Introduction
The interaction of Advanced Glycation End-product (AGE) with Receptor for Advanced Glycation End product (RAGE) on endothelial cells enhances the intracellular Reactive Oxygen Species (ROS) production. Activation of the NADPH Oxidase (NOX) is one of the important mechanisms for ROS generation in the endothelial cells.
Aim
To determine the role of AGE in ROS production via NOX activation in endothelial cells. To assess the ameliorating action of drugs, ramipril, and losartan, as well as the antioxidants, resveratrol and N-Acetyl-Cysteine (NAC) on AGE-mediated effects in endothelial cells.
Materials and Methods
The present experimental in-vitro study was conducted at Department of Biochemistry, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India. The cultured Human Umbilical Vein Endothelial Cells (HUVECs) were treated with advanced glycation end product-bovine serum albumin (AGE-BSA) 200 μg/mL and unmodified BSA in the same concentration for 24 hours. The HUVECs were also co-treated with losartan (5 μM), ramipril (5 μM), resveratrol (5 μM) and NAC (5 μM) with AGE-BSA for 24 hour. ROS generation was assessed by using 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA) method. For the activation of NOX, NOX p47phox subunit mRNA expression was analysed by Real Time Polymerase Chain Reaction (qPCR). Significant difference between the two groups was determined by the Student’s t-test. A value of p<0.05 was considered significant.
Results
A significant increase (p<0.01) in ROS production was observed at a concentration of 200 μg/mL of AGE-BSA as compared with control (cells treated with unmodified BSA). NOX expression in cells was found to be significantly increased (p<0.01) 2-fold at mRNA level after 24 hours treatment with AGE-BSA. Losartan, ramipril, resveratrol and NAC significantly scavenged ROS production, and decreased the AGE-mediated increased NOX mRNA expression was observed.
Conclusion
The present in-vitro study signifies the role of AGE in enhanced ROS generation by activation of NOX in endothelial cells. Losartan, ramipril, resveratrol, and NAC may attenuate the AGE-mediated endothelial dysfunction by counteracting the NOX-mediated increased ROS production.
Introduction
AGE formation is elevated under diabetic conditions and is increasingly evidenced in progression of cardiovascular complications in diabetes mellitus. Hyperglycaemic environment enhances the non-enzymatic reaction of glucose and other reducing sugars with free-amino group containing molecules such as proteins, lipids, and nucleic acids [1] leading to formation of AGE at an accelerated rate, which accumulate in blood and tissues [2]. AGEs have been implicated in the development of vascular complications via interaction with their specific receptor, the RAGE [3]. AGEs found in the plasma, accumulate in the vessel wall. The interaction of AGE with RAGE on the surface of endothelial cells enhances the production of intracellular ROS through activation of the NADPH oxidase that promotes oxidative stress [4]. AGE-induced oxidative stress is a major factor for the activation of downstream consequences such as activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [5] and production of pro-fibrotic factors which may lead to endothelial dysfunction [6].
ROS including superoxide (O2-), hydroxyl (OH), peroxyl (RO2), alkoxyl (RO), and certain non-radicals such as singlet oxygen (O2), and hydrogen peroxide (H2O2) are produced in endothelial cells via various mechanisms including mitochondrial electron transport chain, nitric oxide synthase, xanthine oxidase, cytochrome P-450 enzymes as well as NADPH oxidase (NOX) [7-10]. In turn, when NOX is up-regulated, excess ROS leads to oxidative damage, which results in progression of various diseases like tumor pathogenesis, hypertension and diabetic nephropathy [11-14]. NOXs contains multicomponent enzymatic system that catalyses the conversion of molecular oxygen to superoxide anion radical [15-16]. Among the five known NOX genes (NOX1-5), NOX2 is expressed in phagocytic cells where it serves as the primary source of ROS. Literature suggests that NOX complex is functionally active in human endothelial cells and NOX2 is also expressed in endothelial cells. It has direct influence in the oxidative imbalance, which is considered a pivotal event in endothelial dysfunction [17-19].
Angiotensin Converting Enzyme Inhibitor (ACEI) and Angiotensin II Receptor Blocker (ARB) are anti-hypertensive drugs commonly prescribed for hypertension and Cardiovascular Diseases (CVD). It is reported that these drugs scavenge ROS production and lowers associated intracellular downstream effects [20]. Therefore, the potential role of ACEI and ARB as modulator of oxidative stress is currently an area of active research [21].
Resveratrol is a polyphenol present in grapes, berries, and peanuts that deliberates vaso-protection, improve endothelial function and prevent vascular complications of diabetes in animal models [22-25]. Recent evidence also suggests that resveratrol suppresses ROS production via inhibition of NOX activity [26]. N-acetyl-cysteine (NAC) is a glutathione precursor and thiol-containing free radical scavenger. It protects β-cells from glucose toxicity in both culture and in-vivo conditions, and preserve insulin synthesis and secretion [27]. NAC also acts as an antioxidant by increasing the intracellular concentration of cellular antioxidant capacity and directly scavenging ROS [27-28]. However, studies on exact role and mechanism of resveratrol and NAC on AGE-mediated ROS production are limited.
Hence, the present study was designed to substantiate the notion that AGE increases ROS production in endothelial cells via activation of NOX. In addition, to assess the ameliorating role of ACE inhibitor-ramipril, angiotensin II receptor blocker-losartan, polyphenolic compound-resveratrol and antioxidant-NAC on AGE-mediated vascular effects.
Materials and Methods
The experimental in-vitro study was conducted between January 2015 to February 2016 at Department of Biochemistry, University College of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, India. The present study was approved by Institutional Ethics Committee-Human Research (IEC-HR dated-03/Feb/2014).
AGE-BSA (10 mg/mL) and BSA (10 mg/mL) were purchased from BioVision (Mountain View, CA). The glucose AGE-BSA was produced by reacting BSA with glucose under sterile conditions followed by extensive dialysis and purification steps as per manufacturer’s instructions [29]. Fluorescence of AGE was confirmed by fluorescence spectrophotometry with Ex/Em at 370/440 nm. Glycated BSA showed >50X increase in fluorescence compared to control BSA [30]. Trypsin-EDTA solution, 0.5% gelatin solution, antibiotic solution, dimethyl sulfoxide (DMSO) medium, endothelial growth factors, Foetal Bovine Serum (FBS) were purchased from Himedia Laboratories, Mumbai. Tissue-culture flasks and plates were also supplied by Himedia Laboratories, Mumbai. Ramipril, losartan, NAC and resveratrol were purchased from Sigma-Aldrich, USA.
Culture of Human Umbilical Vein Endothelial Cells
Human Umbilical Vein Endothelial Cells (HiFiTM Umbilical Vein Endothelial cells, product catalogue code:CL002-0.5) were purchased from Himedia Laboratories, Mumbai, India. The endothelial cells were maintained in HiEndoXLTM endothelial cell expansion medium (reduced serum medium), supplemented with endothelial growth factor and 1% antibiotic-antimycotic solution. Cells were incubated in humidified atmosphere with 95% air and 5% carbon dioxide (CO2) at 37°C in an incubation cabinet. For experimental work, endothelial cells were seeded and harvested in gelatin-coated 25-cm2 culture flask and 24-well culture plates. The medium was changed after every 2 days interval. Confluency of HUVECs on different days such as 1st, 3rd and 5th day was checked. Cells were detached using trypsin-EDTA solution on reaching the confluency of 80-90%. Once 80-90% confluency was reached, the cell numbers were counted by cell counter (Invitrogen) and viability of the cells was checked by 3-(4, 5-Dimethylthiazol-2-YI)-2, 5-Diphenyltetrazolium Bromide (MTT) assay. The cultured cells were treated with AGE-BSA (200 μg/mL) and unmodified BSA in the same concentration, and were incubated for 24 hours. Ameliorating effect of losartan, ramipril, resveratrol and NAC were also studied and stock solutions for cell treatment were freshly prepared in DMSO.
Measurement of AGE-Mediated Intracellular ROS Production
The intracellular ROS generation from endothelial cells was assessed by using 2’, 7’-dichlorodihydrofluorescein diacetate (H2DCFDA) from Sigma-Aldrich, USA according to Ledirac N et al., method [31]. Briefly, the HUVECs were treated with AGE-BSA (200 μg/mL), unmodified BSA (200 μg/mL), losartan (5 μM), ramipril (5 μM), resveratrol (5 μM) and NAC (5 μM) in the presence of 100 μM H2DCFDA (the stock solution was made in ethanol to maintain the final concentration in the medium at 0.33%) for 90 minutes at 37°C. However, cells were already pre-incubated with losartan (5 μM) ramipril (5 μM), resveratrol (5 μM) and NAC (5 μM) for 24 hours. After the incubation period, cells were washed twice with cold Phosphate Buffered Saline (PBS), and scraped in potassium buffer (10 mM pH 7.4) /methanol (v/v) completed with Triton X-100 (0.1%). An aliquot of 100 μL was incubated in a black 96-well plate, and relative fluorescence intensity was determined by Multimode Plate Reader (Molecular Devices, California, USA) at an excitation wavelength of 488 nm and an emission wavelength of 520 nm.
AGE-Mediated NADPH Oxidase p47Phox mRNA Expression
Total RNA was extracted from HUVECs by using Trizol (Invitrogen, Carlsbad, CA) method. The concentration of total RNA and 260/280 absorbance ratio were measured by Nano DropTM spectrophotometer (Thermo Fisher ScientificTM). c-DNA was synthesised using a Maxima First Strand Synthesis Kit (Fermentas, Germany). Quantitative Real Time PCR (qPCR) reaction was performed on the CFX ConnectTM Real Time PCR detection system (Bio-Rad, USA) to measure mRNA expression of NADPH oxidase p47phox using SYTO9 fluorescent dye. The PCR reaction mixture comprised of 4 μL of template c-DNA, 10 μL of Pyrostart Fast PCR master mix (Fermentas, Germany) and 10 pmoL of each primer of both target gene and housekeeping gene. The sequence of each primer is mentioned in [Table/Fig-1].
Forward and reverse primer sequence of both target gene and housekeeping gene.
| Target | Direction | Primer sequence |
|---|
| NADPH gene | forward: | 5’-GCTCCCCACGGACAACCAGAC 3’ |
| reverse: | 5’ TCTTCTCCACGACC TCCACCAC 3’ |
| 18S rRNA | forward: | 5’CGGAGGTTCGAAGACGATCAGATA 3’ |
| reverse: | 5’ TTGGTTTCCCGGAAGCTGCC-3’ |
The genes were amplified by 35 cycles of 95°C for 45 seconds (denaturation step), 61°C for 30 seconds (annealing step) and final extension step for 60 seconds at 72°C. During thermal cycling, emission from each sample was recorded, and Rotor Gene Q software was used to process the raw fluorescence data to produce threshold cycle (Ct) values. The housekeeping gene (18S rRNA) was used for internal normalisation. The relative fold change was calculated by the 2-ΔΔCt method [32].
Statistical Analysis
The data analysis was carried out using standard statistical methods (SPSS software version 20.0). Data were expressed as Mean±Standard Error of Mean (SEM). Statistical significant difference between two groups was determined by the Student’s Two-tailed t-test. A value of p<0.05 was considered significant.
Results
b>HUVECs Morphology and Confluency Status
Confluency of HUVECs on 1st, 3rd and 5th day are presented in [Table/Fig-2].
Human Umbilical Vein Endothelial cells (HUVECs) confluence on 1st, 3rd and 5th day.
AGE-Mediated Intracellular ROS Generation
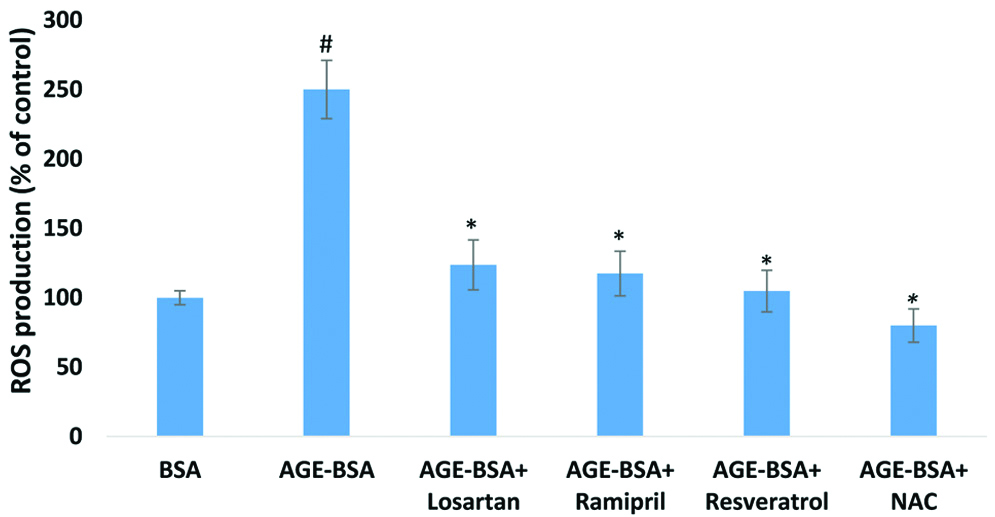
AGE-BSA treated HUVECs led to significantly increased ROS production (2.5-fold; p<0.01) in comparison to unmodified BSA. However, unmodified BSA had no effect on ROS production [Table/Fig-3].
Effect of AGE-BSA on ROS production in HUVECs.
HUVECs were treated with AGE-BSA (200 μg/mL) and unmodified BSA (control) in same concentration. Drugs and antioxidants effectively decreased the AGE-induced ROS production in HUVEC. The concentrations of drugs and antioxidants which were used to attenuate AGE-mediated ROS production respectively, 5 μM of losartan, 5 μM of ramipril, 5 μM of resveratrol and 5 μM of NAC. Data represents mean±SEM of three independent experiments done in triplicate. #p<0.01 by two-tailed t-test for comparison with the cells treated with unmodified BSA. *p<0.01 by two-tailed t-test for comparison with the cells treated with AGE-BSA
Ameliorating Effect of Drugs and Antioxidants on AGE-Mediated ROS Production
The drugs and antioxidants were co-treated with AGE-BSA to find out the degree of attenuation on ROS production [Table/Fig-3]. This ROS production was significantly (p<0.01) attenuated 50.5% by losartan, 53% by ramipril, 58% by resveratrol and 68% by NAC in comparison to cells treated with only AGE-BSA.
NADPH Oxidase (p47phox subunit) Expression in HUVECs
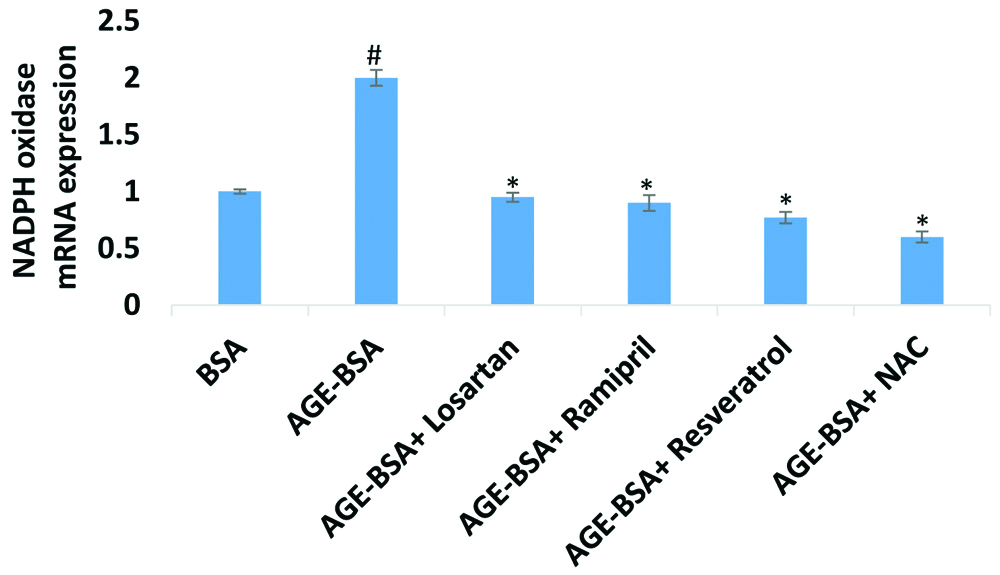
AGE-BSA concentration (200 μg/mL) was significantly increased (p<0.01) NADPH oxidase p47 phox subunit mRNA expression in HUVECs at 24 hour incubation in treated cells as compared to control (cells treated with unmodified BSA). AGE-BSA had increased p47phox mRNA expression 2-fold as compared to control [Table/Fig-4].
AGE-BSA mediated NADPH oxidase mRNA expression in HUVECs.
HUVECs were treated with concentration of AGE-BSA (200 μg/mL) and unmodified BSA (control) in same concentration. Drugs and antioxidants effectively decreased the AGE-induced NADPH oxidase expression in HUVECs. The concentrations of Drugs and antioxidants which were used respectively, 5 μM of losartan, 5 μM of ramipril, 5 μM of resveratrol and 5 μM of NAC. Data represents mean±SEM of three independent experiments done in triplicate. #p<0.01 by two-tailed t-test for comparison with the cells treated with unmodified BSA. *p<0.01 by two-tailed t-test for comparison with the cells treated with AGE-BSA
Ameliorating Effect of Drugs and Antioxidants on AGE-Mediated NADPH Oxidase mRNA Expression in HUVECs
HUVECs co-treated with losartan (5 μM) showed significant (53%) decrease in expression of NADPH oxidase whereas when cells co-treated with ramipril (5 μM) showed (55%) decreased expression of NADPH oxidase. Cells co-treated with antioxidants resveratrol (5 μM) and NAC (5 μM) showed significant inhibition of NADPH oxidase expression. AGE-mediated expression of NADPH oxidase in HUVECs was decreased by resveratrol and NAC respectively, 60% and 70%. The results indicate that NAC is more effective in lowering AGE-mediated NADPH oxidase mRNA expression [Table/Fig-4].
Discussion
This study was carried out to determine the effect of AGE-BSA on endothelial cells. The in-vitro result showed that exposure of HUVECs to AGE-BSA stimulates increased ROS production as compared to control (cells treated with unmodified BSA). These results were similar to various other studies that reported increased AGE-induced ROS production in human endothelial cells [33-34]. AGE-mediated enhanced production of ROS is associated with the activation of NF-κB transcription factor, pro-fibrotic factors such as TGF-β1 and inflammatory markers [35]. Activation of these downstream signaling pathways caused by enhanced ROS production may help in explaining the AGE-associated endothelial dysfunction [6].
To assess whether AGE-mediated enhanced ROS production occurs via NOX pathway, AGE-treated HUVECs were evaluated for p47phox NOX mRNA expression. A significant two-fold increase in mRNA expression was found in AGE-BSA treated cells as compared to control cells. Wautier MP et al., also reported that activation of NOX in endothelial cells is associated with oxidative stress and AGE may be one of the important factors for vascular damage in diabetes by enhancing the ROS production in endothelial cells [36]. NOX-induced ROS production is also involved in pathogenesis of various diseases including inflammation, diabetic nephropathy, endocrine disruption, endothelial dysfunction and kidney damage [37-38]. Greiber S et al., demonstrated ROS production by the expression of p47phox NOX, where ROS was primarily generated by NOX, and the NOX subunits such as p22phox, p47phox, NOX2, and p67phox were expressed in podocytes [39]. The translocation and binding of p47phox, a cytoplasmic subunit of NOX with membrane complex of NOX2 and p22phox are the key events that lead to the activation of NOX and generation of ROS [40].
In the present study, efficacy of drugs like; ramipril and losartan and antioxidants such as NAC and resveratrol in attenuation of AGE-mediated effects were also examined. Evidence indicates that the effect of ACE inhibitor (ACEI) and AT1R blocker (ARB) can be attributed to direct inhibition of NOX activity, and antioxidant properties of these drugs. It has also been suggested that the beneficial effect of antihypertensive drugs such as ACEI and ARB may be mediated, in part, by decreasing vascular oxidative stress [17, 41-42]. Fortuno J et al., also reported that losartan metabolite EXP3179 blocked NOX-mediated ROS generation which confers losartan have specific capacity to reduce oxidative stress through NOX regulation [43]. Protective role of antioxidants against AGE effects are well-studied and shows the involvement of resveratrol in inhibition of oxidative stress in aorta of diabetic mice and modification of human B lymphocyte proliferation and apoptosis [44]. Furthermore, NAC hinders the production of intracellular ROS and activation of JNK and p38 MAPK pathways, and thereby inhibits the process of apoptosis of AGE-treated HUCLs [45].
Limitation and Future Recommendation
The potential mechanism and signaling pathways that describe the AGE-RAGE-mediated endothelial dysfunction were not carried out in this study. Further, in-vitro research is required to investigate and uncover the possible mechanisms.
Conclusion
The present study demonstrated that AGE-induced ROS production mediated by increased NADPH oxidase activity could be responsible for the activation of intracellular downstream signaling pathways, which might lead to endothelial dysfunction. Losartan, ramipril, resveratrol and NAC could ameliorate AGE-mediated endothelial dysfunction by attenuation of both NADPH oxidase activation and its associated intracellular oxidative stress.
[1]. Helou C, Marier D, Jacolot P, Abdennebi-Najar L, Niquet-Léridon C, Tessier FJ, Microorganisms and Maillard reaction products: a review of the literature and recent findingsAmino Acids 2014 46(2):267-77.10.1007/s00726-013-1496-y23588491 [Google Scholar] [CrossRef] [PubMed]
[2]. John WG, Lamb EJ, The Maillard or browning reaction in diabetesEye 1993 7:230-37.10.1038/eye.1993.557607341 [Google Scholar] [CrossRef] [PubMed]
[3]. Yan SF, Ramasamy R, Schmidt AM, The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculatureCirc Res 2010 106:842-53.10.1161/CIRCRESAHA.109.21221720299674 [Google Scholar] [CrossRef] [PubMed]
[4]. Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expressionJ Biol Chem 1999 274:31740-49.10.1074/jbc.274.44.3174010531386 [Google Scholar] [CrossRef] [PubMed]
[5]. Dąbek J, Kułach A, Gąsior Z, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB): a new potential therapeutic target in atherosclerosis?Pharmacol Rep 2010 62(5):778-83.10.1016/S1734-1140(10)70338-8 [Google Scholar] [CrossRef]
[6]. Bierhaus A, Illmer T, Kasper M, Luther T, Quehenberger P, Tritschler H, Advanced glycation end product (AGE)-mediated induction of tissue factor in cultured endothelial cells is dependent on RAGECirculation 1997 96:2262-71.10.1161/01.CIR.96.7.22629337199 [Google Scholar] [CrossRef] [PubMed]
[7]. Xiao W, Peng Y, Liu Y, Li Z, Li S, Zheng X, HSCARG Inhibits NADPH Oxidase Activity through Regulation of the Expression of p47phoxPLoS One 2013 8(3):e5930110.1371/journal.pone.005930123527155 [Google Scholar] [CrossRef] [PubMed]
[8]. Droge W, Free radicals in the physiological control of cell functionPhysiol Rev 2001 82:47-95.10.1152/physrev.00018.200111773609 [Google Scholar] [CrossRef] [PubMed]
[9]. Bedard K, Krause KH, The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiologyPhysiol Rev 2007 87:245-313.10.1152/physrev.00044.200517237347 [Google Scholar] [CrossRef] [PubMed]
[10]. Riganti C, Gazzano E, Polimeni M, Costamagna C, Bosia A, Diphenyleneiodonium inhibits the cell redox metabolism and induces oxidative stressJ Biol Chem 2004 279:47726-31.10.1074/jbc.M40631420015358777 [Google Scholar] [CrossRef] [PubMed]
[11]. Guijarro MV, Leal JF, Blanco-Aparicio C, Alonso S, Fominaya J, Lleonart M, MAP17 enhances the malignant behavior of tumor cells through ROS increaseCarcinogenesis 2007 28:2096-104.10.1093/carcin/bgm12417548903 [Google Scholar] [CrossRef] [PubMed]
[12]. Bechtel W, Bauer G, Catalase protects tumor cells from apoptosis induction by intercellular ROS signalingAnticancer Res 2009 29:4541-57. [Google Scholar]
[13]. Paravicini TM, Touyz RM, NADPH oxidases, reactive oxygen species, and hypertension: clinical implications and therapeutic possibilitiesDiabetes Care 2008 31:S170-S180.10.2337/dc08-s24718227481 [Google Scholar] [CrossRef] [PubMed]
[14]. Fridlyand LE, Philipson LH, Oxidative reactive species in cell injury: Mechanisms in diabetes mellitus and therapeutic approachesAnn N York Acad Sci 2005 1066:136-51.10.1196/annals.1363.01916533924 [Google Scholar] [CrossRef] [PubMed]
[15]. Cathcart MK, Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosisArterioscler, Thromb, Vasc Biol 2004 24:23-28.10.1161/01.ATV.0000097769.47306.1214525794 [Google Scholar] [CrossRef] [PubMed]
[16]. Groemping Y, Rittinger K, Activation and assembly of the NADPH oxidase: a structural perspectiveBiochem J 2005 386:401-16.10.1042/BJ2004183515588255 [Google Scholar] [CrossRef] [PubMed]
[17]. Chhabra N, Endothelial dysfunction- A predictor of atherosclerosisIJMU 2009 4(1):33-41.10.4314/ijmu.v4i1.39872 [Google Scholar] [CrossRef]
[18]. Drummond GR, Selemidis S, Griendling KK, Sobey CG, Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targetsNat Rev Drug Discov 2011 10:453-71.10.1038/nrd340321629295 [Google Scholar] [CrossRef] [PubMed]
[19]. Al-Medhi AB, Zhao G, Dodia C, Tozawa K, Costa K, Muzykantov V, Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+Circ Res 1998 83:730-37.10.1161/01.RES.83.7.7309758643 [Google Scholar] [CrossRef] [PubMed]
[20]. Fiordaliso F, Cuccovillo I, Bianchi R, Bai A, Doni M, Salio M, Cardiovascular oxidative stress is reduced by an ACE inhibitor in a rat model of streptozotocin-induced diabetesLife Sci 2006 79:121-29.10.1016/j.lfs.2005.12.03616445948 [Google Scholar] [CrossRef] [PubMed]
[21]. Touyz RM, Reactive Oxygen Species, Vascular Oxidative Stress, and Redox Signaling in HypertensionHypertension 2004 44:248-52.10.1161/01.HYP.0000138070.47616.9d15262903 [Google Scholar] [CrossRef] [PubMed]
[22]. Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K, Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in ratsPharmacology 2006 76:69-75.10.1159/00008972016286809 [Google Scholar] [CrossRef] [PubMed]
[23]. Su HC, Hung LM, Chen JK, Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic ratsAm Physiol Endocrinol Metab 2006 290:E1339-E46.10.1152/ajpendo.00487.200516434553 [Google Scholar] [CrossRef] [PubMed]
[24]. Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenaseFree Radic Biol Med 2007 43:720-29.10.1016/j.freeradbiomed.2007.05.00417664136 [Google Scholar] [CrossRef] [PubMed]
[25]. Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient miceDiabetes 2006 55:2180-91.10.2337/db05-118816873680 [Google Scholar] [CrossRef] [PubMed]
[26]. Chow SE, Hshu YC, Wang JS, Chen JK, Resveratrol attenuates oxLDL-stimulated NADPH oxidase activity and protects endothelial cells from oxidative functional damagesJ Appl Physiol 2007 102:1520-27.10.1152/japplphysiol.00881.200617194732 [Google Scholar] [CrossRef] [PubMed]
[27]. Haber CA, Lam TK, Yu Z, Gupta N, Goh T, Bogdanovic E, N-acetylcysteine and taurine prevent hyperglycemia-induced insulin resistance in vivo: Possible role of oxidative stressAm J Physiol Endocrinol Metab 2003 285:E744-E53.10.1152/ajpendo.00355.200212799318 [Google Scholar] [CrossRef] [PubMed]
[28]. Da Ros R, Assaloni R, Ceriello A, Antioxidant therapy in diabetic complications: what is new?Curr Vasc Pharmacol 2004 2:335-41.10.2174/157016104338553815320813 [Google Scholar] [CrossRef] [PubMed]
[29]. Valencia JV, Mone M, Koehne C, Rediske J, Hughes TE, Binding of receptor for advanced end products (RAGE) ligands is not sufficient to induce inflammatory signals: lack of activity of endotoxin-free albumin-derived advanced glycation end productsDiabetologia 2004 47:844-52.10.1007/s00125-004-1392-915127201 [Google Scholar] [CrossRef] [PubMed]
[30]. Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kineticsAnal Biochem 2004 324(1):68-78.10.1016/j.ab.2003.09.01314654047 [Google Scholar] [CrossRef] [PubMed]
[31]. Ledirac N, Anthericu S, d’Uby AD, Caron JC, Rahmani R, Effect of organochlorine insecticides on MAP kinase pathways in human HaCaT keratinocytes: key role of reactive oxygen speciesToxicol. Sci 2005 86(2):444-52.10.1093/toxsci/kfi19215888667 [Google Scholar] [CrossRef] [PubMed]
[32]. Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))Methods 2001 25(4):402-08.10.1006/meth.2001.126211846609 [Google Scholar] [CrossRef] [PubMed]
[33]. Goldin A, Beckman JA, Schmidt AM, Creager MA, Advanced glycation end products: sparking the development of diabetic vascular injuryCirculation 2006 114:597-605.10.1161/CIRCULATIONAHA.106.62185416894049 [Google Scholar] [CrossRef] [PubMed]
[34]. He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE, Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetesNutr, Metab Cardiovasc Dis 2011 21:277-85. [Google Scholar]
[35]. Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K, Diabetes and Kidney Disease: Role of Oxidative StressAntioxid Redox Signal 2016 25(12):657-84.10.1089/ars.2016.666426906673 [Google Scholar] [CrossRef] [PubMed]
[36]. Wautier MP, Chappey O, Corda S, Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGEAm J Physiol Endocrinol Metab 2001 280:E685-94.10.1152/ajpendo.2001.280.5.E68511287350 [Google Scholar] [CrossRef] [PubMed]
[37]. Deng J, Wang X, Qian F, Vogel S, Xiao L, Ranjan R, Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expressionJ Immunol 2012 188:5734-40.10.4049/jimmunol.110132322547702 [Google Scholar] [CrossRef] [PubMed]
[38]. Liu GC, Fang F, Zhou J, Koulajian K, Yang S, Lam L, Deletion of p47phox attenuates the progression of diabetic nephropathy and reduces the severity of diabetes in the Akita mouseDiabetologia 2012 55:2522-32.10.1007/s00125-012-2586-122653270 [Google Scholar] [CrossRef] [PubMed]
[39]. Greiber S, Münzel T, Kästner S, Müller B, Schollmeyer P, Pavenstädt H, NAD(P)H oxidase activity in cultured human podocytes: effects of adenosine triphosphateKidney Int 1998 53:654-63.10.1046/j.1523-1755.1998.00796.x9507211 [Google Scholar] [CrossRef] [PubMed]
[40]. Chen S, Meng XF, Zhang C, Role of NADPH Oxidase-Mediated Reactive Oxygen Species in Podocyte InjuryBioMed Res Int 2013 :1-7.10.1155/2013/83976124319690 [Google Scholar] [CrossRef] [PubMed]
[41]. Ghiadoni L, Magagna A, Versari D, Kardasz I, Huang Y, Taddei S, Different effect of antihypertensive drugs on conduit artery endothelial functionHypertension 2003 41:281-86.10.1161/01.HYP.0000070956.57418.2212719441 [Google Scholar] [CrossRef] [PubMed]
[42]. Yoshida J, Yamamoto K, Mano T, Sakata Y, Nishikawa N, Nishio M, AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failureHypertension 2004 43:686-91.10.1161/01.HYP.0000118017.02160.fa14757777 [Google Scholar] [CrossRef] [PubMed]
[43]. Fortuno J, Bidegain PA, Robador Hermida J, López-Sagaseta J, Beloqui O, Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertensionHypertension 2009 54:744-50.10.1161/HYPERTENSIONAHA.109.12935319687351 [Google Scholar] [CrossRef] [PubMed]
[44]. Zunino SJ, Storms DH, Resveratrol alters proliferative responses and apoptosis in human activated B lymphocytes in vitroJ Nutr 2009 139:1603-08.10.3945/jn.109.10506419549761 [Google Scholar] [CrossRef] [PubMed]
[45]. Shi L, Yu X, Yang H, Wu X, Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathwaysPLoS ONE 2013 8:e6678110.1371/journal.pone.006678123776698 [Google Scholar] [CrossRef] [PubMed]