AHSCT is a curative treatment for many malignant and non-malignant haematologic disorders such as aplastic anaemia/marrow-failure states plus a continuously expanding list of auto-immune, inherited metabolic and immuno-deficiency diseases [1].
The outcome of patients receiving AHSCT is measured in terms of overall survival and disease-free survival. These endpoints represent a summary of many events related to GVHD, treatment related complications and disease relapse [2]. Patients receiving HSCT require essential RBC and platelet transfusion support until engraftment occurs and sufficient haematopoiesis is achieved in all lineages. The requirement criteria of transfusion support for patients receiving HSCT as regard to duration, frequency and type of blood products needed are variable depending on several factors such as the nature of the disease, the source of the stem cells, the conditioning pre-transplant regimen, and patient factors during the post-transplantation period [3,4].
A transfusion threshold at a Hb level of 7 or 8 g/dL is well accepted among transplant centers, which mirrors the current AABB guidelines for critically ill, hospitalised patients while platelet transfusions is minimised by adhering to evidence-based guidelines, using a platelet count of 10,000/μL as a threshold for administering platelets to a non-bleeding patients [4]. Transfusion related complications are more frequently seen in the patient population receiving AHSCT. Standard transfusion reactions, such as allergic or febrile non-haemolytic reactions, are frequently seen in this heavily transfused patient population [5]. Some of these complications occur due to activation of the lymphocytes within the transplant against the recipient, leading to Transfusion Associated Graft Versus Host Disease (TA-GVHD) and Passenger Lymphocyte Syndrome (PLS) [6]. Logan AC et al., performed a retrospective, multivariate analysis that showed among patients who underwent AHSCT, ABO mismatch between allo-HSCT donor and recipient has been associated with an increased risk of GVHD in some patient populations, however not in others [7].
In addition, blood transfusion related iron overload, is commonly seen in those patients undergoing HSCT. Several previous studies have documented the adverse prognostic impact of iron overload in such patients as it increases the risk of acute GVHD, the incidence of blood stream infections and sinusoidal obstruction syndrome of the liver [8]. However, the prognostic impact of blood transfusion on the post-transplant outcome was seldom investigated.
Here, the aim of this study was to evaluate the impact of RBC and/or platelet transfusion on the incidence of infection, risk of development of acute and chronic GVHD and overall survival in patients undergoing AHSCT.
Materials and Methods
The present study was conducted on 50 adult patients with primary malignant haematological diseases who achieved complete remission and underwent AHSCT from fully Human Leucocyte Antigens (HLA) matched sibling donor at Ain Shams university bone marrow transplantation unit, between 2015 to 2018.
A retrospective analysis was done for the clinical and laboratory data of included patients to explore the impact of amount of transfused units of blood products on post-transplantation sequels.
The study excluded the data of patients who had any of the following conditions: patients in relapse or partial remission, patients with platelet refractoriness, patients with ABO mismatched AHSCT, patients who did not achieve complete chimera (>90% donor cells) before day 30 or engraftment (neutrophil count >0.5x 109 /L) up to day 35, patients whose disease relapsed until day 60 and patients who did not survive until day 100.
The research protocol for data analysis of this study was reviewed and approved by Ain Shams University Ethical Committee for research and it was in accordance with the 1964 declaration of Helsinki and its later amendments. Written informed consent was obtained from all included patients in the study.
Patients Criteria
Diagnosis of 50 included patients varied between Acute Myeloid Leukaemia (AML), Acute Lymphoblastic Leukaemia (ALL), biphenotypic acute leukaemia, and high risk Myelo Dysplastic Syndrome (MDS). Patients with AML and high risk MDS were given Conditioning regimens pre-transplant included fludarabine/busulphan protocol. It was constituted of fludarabine 30 mg/m2/day IV (day-6 to -3), busulphan 1 mg/kg/6 hours orally (day-6 to -3) and patients with biphenotypic acute leukaemia received fludarabine/busulphan/cytosine arabinoside /vepeside protocol which is composed of five days of intravenous fludarabine at a dose of 30 mg/m2/day (day -6 to -2), cytosine arabinoside given at a dose of 1200 mg/m2/day intravenously (at day -6 and -5), busulphan at a dose of 4.5 mg/kg/day (at day -4 and -2) orally, and etoposide at a dose of 6 mg/kg/day intravenously (from day -6 to day -2), while patients with ALL received TBI/cyclophosphamide protocol, constituted of fractionated 12 Gray TBI over 3 days followed by cyclophosphamide 120 mg/kg over 2 days as a pre-transplant conditioning regimen.
The data of the selected patients were divided in two groups according to the amount of transfused units of blood products, Group (I) low transfusion group including 30 patients who received less than 10 units and Group (II) the high transfusion group including 20 patients who received more than 10 units.
RBC and Platelet Transfusions Criteria
All RBC and platelet units were irradiated and RBCs were used within 28 days of irradiation. All transfused units were provided by Ain shams university hospital blood bank. Transfusion threshold for one platelet apheresis unit was platelet count <10,000/μL or at the time of bleeding. While the transfusion threshold for 2 RBCs units was haemoglobin level 7-8 gm/dL or in case of haemorrhage-induced anaemia to maintain haemoglobin level >8 gm/dL. No erythropoiesis stimulating agents were used in these patients.
Methodology and Study Endpoint
In this retrospective study, all relevant data were gathered from patients records during the first year after AHSCT, the collected data included the number and type of units received (Packed RBCs or platelets) and the time of receiving the transfusion with respect to transplantation. Also, patient records during post-transplant period were reviewed with regards to development of Infectious episodes (bacterial, viral, parasitic or fungal) other than neutropenia, development of GVHD (either acute or chronic, time of presentation, type of presentation) and overall survival time.
Statistical Analysis
Data were analysed using Statistical Program for Social Science (SPSS) version 20.0. Quantitative data were expressed as mean±Standard Deviation (SD). Qualitative data were expressed as frequency and percentage. Independent-samples t-test of significance was used when comparing between two means. Chi-square (χ2) test was used to compare proportions between two qualitative parameters; p-value>0.05 non-significant, p≤0.05 significant, p≤0.01 highly significant. A Kaplan-Meir curve was plotted to calculate the cumulative survival time.
Results
A total of 50 patients data were included; there were 26 males and 24 females with an age range between 17 and 60 years. The mean age of group 1 was 35.1±12.85 and of group 2 was 35.3±11.85. Insignificant differences were found between both groups regarding age, gender, primary malignant haematological disease, pre-transplant conditioning regimen and GVHD prophylaxis regimen.
Regarding the type of units used, 3 out of 30 patients (10%) in group 1 needed only RBCs, 7 patients (23.3%) needed only platelet transfusion and 10 patients (33.3%) needed both platelet and packed RBCs. While in group 2, all patients (100%) needed transfusion of both blood components with significant differences (p<0.001) [Table/Fig-1].
Comparison between groups according to type of units.
| Type of units | Group I (<10 units) (N=30) | Group II (>10 units) (N=20) | Chi-square test |
|---|
| No. | % | No. | % | χ2 | p-value |
| Non | 10 | 33.3% | 0 | 0.0% | 9.706 | <0.001 |
| RBCs | 3 | 10.0% | 0 | 0.0% | 1.404 | 0.236 |
| PLTS | 7 | 23.3% | 0 | 0.0% | 5.810 | 0.016 |
| RBCS+PLTS | 10 | 33.3% | 20 | 100.0% | 27.097 | <0.001 |
Non: Patients with no transfusion requirements; PLTS: Platelets transfusion; RBCs: packed Red blood cells transfusion
Impact of Blood Transfusion on Development of Infection
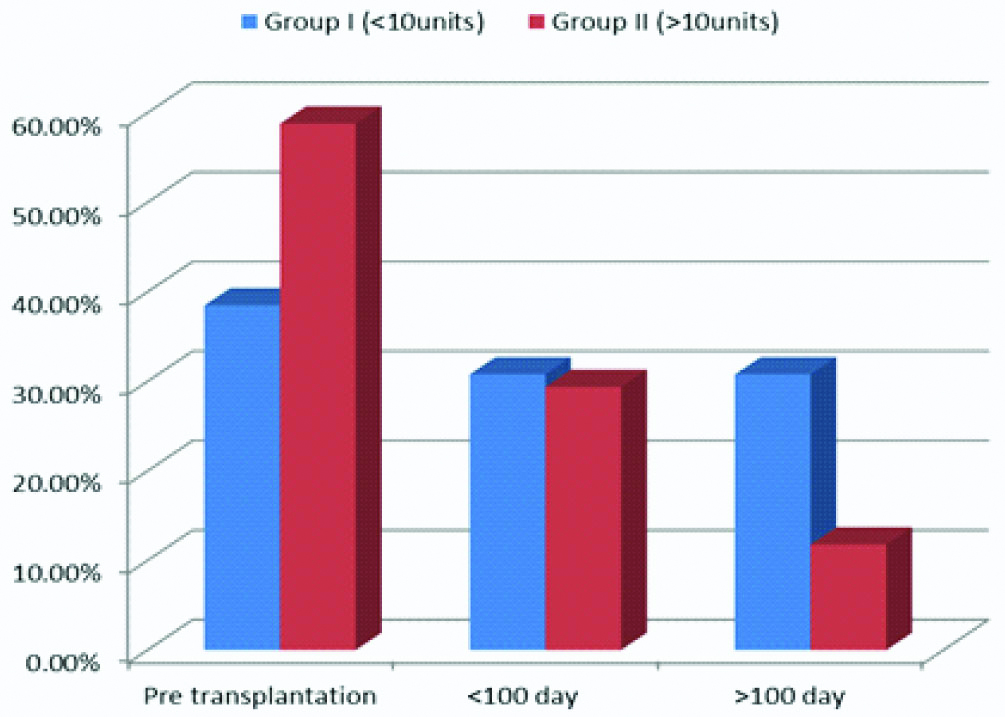
Twenty four patients out of 50 (48%) experienced a total of 30 infectious episodes during a period of one year after transplant. Group 1 patients experienced 43.3% of the total infectious episodes while 56.7% of infectious episodes experienced by group 2 patients. There was a statistically significant difference between the two groups in developing infections p=0.006. There was a statistically significant difference between the groups according to incidence of viral HBV [Table/Fig-2,3].
Comparison between groups according to infection.
| Infection | Group I (<10 units) (N=30) | Group II (>10 units) (N=20) | Chi-square test |
|---|
| No. | % | No. | % | χ2 | p-value |
|---|
| Non | 20 | 73.3% | 6 | 30.0% | 7.457 | 0.006 |
| Bacterial | 2 | 6.7% | 2 | 10.0% | 0.014 | 0.915 |
| Viral-HCV | 1 | 3.3% | 2 | 10.0% | 0.274 | 0.601 |
| Viral-HBV | 2 | 6.7% | 6 | 30.0% | 3.991 | 0.046 |
| Viral-CMV | 1 | 3.3% | 1 | 5.0% | 0.191 | 0.662 |
| Fungal | 1 | 3.3% | 0 | 0.0% | 0.046 | 0.829 |
| Bacterial+Viral-HCV | 1 | 3.3% | 1 | 5.0% | 0.191 | 0.662 |
| Bacterial+Viral-HBV | 1 | 3.3% | 0 | 0.0% | 0.046 | 0.829 |
| Viral-HCV+Fungal | 0 | 0.0% | 1 | 5.0% | 0.042 | 0.837 |
| Viral-CMV+Fungal | 1 | 3.3% | 1 | 5.0% | 0.191 | 0.662 |
Non: Patients with no infectious episodes
Bar chart between groups according to time of infection.
Impact of Blood Transfusion on Development of GVHD
Of the 50 patients who achieved donor engraftment, the number of patients who did not developed GVHD was 28 out of 30 in group 1 (93.3%) and 13 out of 20 in group 2 (65%), with a significant difference (p=0.029) but there was no significant difference between the two groups regarding the organs affected. Acute GVHD developed in one of 30 patients (3.3%) in group 1 and 3 of 20 (15%) in group 2. Patients who achieved durable engraftment and survived for 100 days, chronic extensive GVHD developed in one patient (3.3%) in the low transfusion group and 4 of 20 (20%) in the higher transfusion group [Table/Fig-4,5].
Comparison between groups according to GVHD.
| GVHD | Group I (<10 units) (N=30) | Group II (>10units) (N=20) | Chi-square test |
|---|
| No. | % | No. | % | χ2 | p-value |
|---|
| Non | 28 | 93.3% | 13 | 65.0% | 4.731 | 0.029 |
| GIT | 0 | 0.0% | 2 | 10.0% | 1.063 | 0.302 |
| Liver | 1 | 3.3% | 1 | 5.0% | 0.191 | 0.662 |
| Skin+GIT | 1 | 3.3% | 2 | 10.0% | 0.137 | 0.711 |
| Liver+GIT | 0 | 0.0% | 1 | 5.0% | 0.042 | 0.837 |
| Skin+GIT+liver | 0 | 0.0% | 1 | 5.0% | 0.042 | 0.837 |
Comparison between groups according to type of GVHD.
| Type of GVHD | Group I (<10 units) (N=30) | Group II (>10 units) (N=20) | Chi-square test |
|---|
| No. | % | No. | % | χ2 | p-value |
|---|
| Acute | 1 | 3.3% | 3 | 15% | 0.927 | 0.335 |
| Chronic | 1 | 3.3% | 4 | 20% | 2.098 | 0.148 |
| No | 28 | 93.3% | 13 | 65% | 4.732 | 0.029 |
Impact of Blood Transfusion on Overall Survival
Both groups showed a variable difference in overall survival in first year post-transplant. The first year survival was 46.7% in group 1 and 25% in group 2 with a significant difference (p=0.021). No statistically significant difference was found between groups according to median of survival (two years) however low transfusion group still had increased survival [Table/Fig-6,7].
Kaplan-Meir curve: Survival between group I and group II.
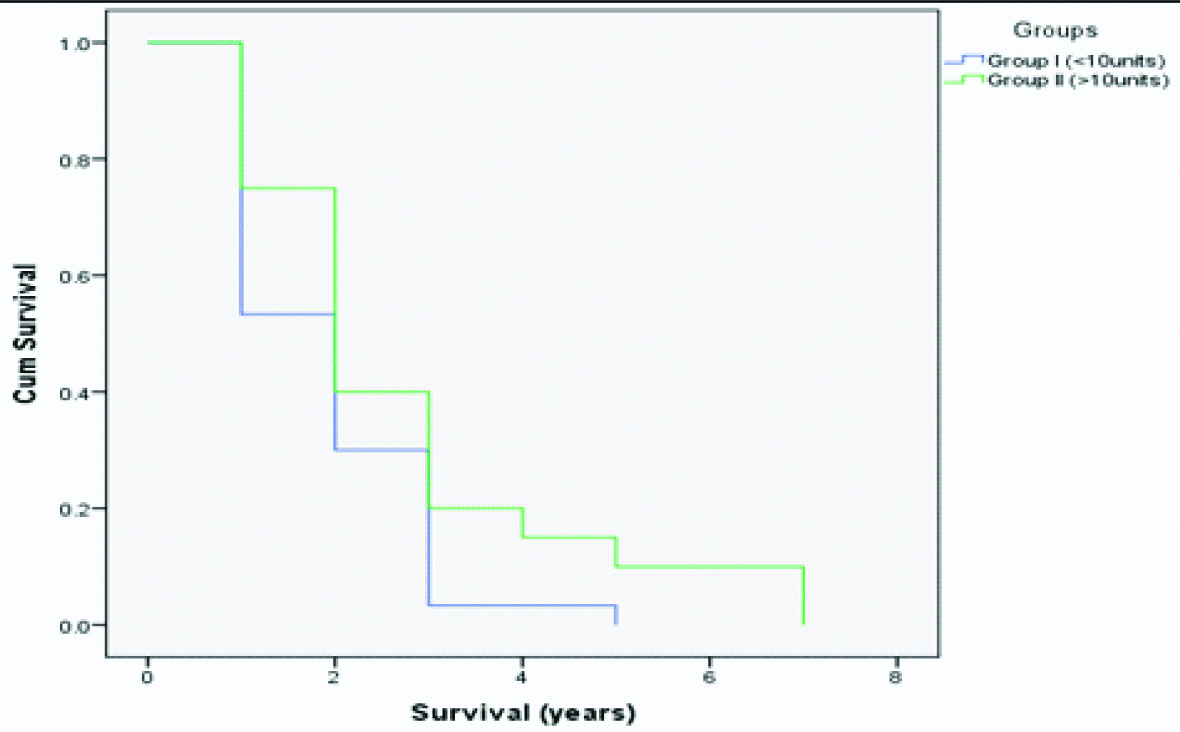
Survival between group I and group II.
| Survival | Median estimate | Std. error | 95% C.I. | Chi-square | Sig. |
|---|
| Lower | Upper |
|---|
| Group I (<10 units) | 53.3% | 2.00 | 0.23 | 1.45 | 2.35 | 2.007 | 0.098 |
| Group II (>10 units) | 40.0% | 2.00 | 0.31 | 1.39 | 2.61 |
| Overall | 48.0% | 2.00 | 0.24 | 1.53 | 2.47 |
Discussion
Despite advances in transplantation practice, morbidities and mortality remains a major hurdle to improving HSCT outcome [9]. In light of the fact that the impact of blood transfusion on clinical outcomes in patients undergoing AHSCT is still poorly understood. This study was done to gain more insight and knowledge about the association between the number of RBC and /or platelet units transfused with the incidence of infection, development of GVHD and overall survival. The current study found that the incidence of developing infection was significantly higher among the high transfusion group (60% of this group experienced at least one infectious episode during the investigated period) than that in the low transfusion group (p=0.006) which may be attributed to transfusion transmitted infection and the state of transfusion induced iron overload that increase the risk of blood stream infection.
Many previous studies were in agreement with the current study as they found that the total volume of RBC and platelet units transfused positively correlated with the infection rate (p<0.05) and restriction of transfusion in HSCT patients is associated with a lower incidence of infection than in patients with high transfusion burden and they attributed their finding to the iron overload in such patients [10,11]. The present study found a significant difference between the high and low transfusion groups regarding the incidence of Hepatitis B virus (HBV) which was higher in the high transfusion group (30%) than in the low transfusion group (6.7%). Furthermore, the incidence of Hepatitis C virus (HCV) was also higher in the high transfusion group 10% than in low transfusion group 3.3%. The incidence of HBV and HCV was higher in the index study than the results reported by Francisci D et al., who found that the prevalence of HBV was 15.6% and that of HCV was 3.7% in patients undergoing AHSCT [12]. While in the current study, the incidence of CMV was 6.6% in low transfusion group and 10% in high transfusion group with no significant difference between the two groups. A higher incidence was reported by Slade M et al., who found the incidence of CMV infection was (15%) and higher incidence of viremia was observed [13].
The results of the present study demonstrated that the incidence of GVHD was significantly higher in high transfusion group than in low transfusion group. The incidence of both acute GVHD and chronic GVHD were higher in high transfusion group, these results were comparable to other published results by Lee S et al., who found that the number of pre-transplantation RBC transfusions was associated with aGVHD in AHSCT patients and they suggest that these results are due to the iron overload state in high transfusion group [14]. Another study by Hosoba S et al., who demonstrated positive association between the number of RBC units transfused with the incidence of severe aGVHD in patients undergoing AHSCT however they attributed that the increased incidence of development of aGVHD to the associated anaemia [15]. This association of RBC transfusions with aGVHD was also agreed by Hendrickson JE et al., who thought that transfused RBCs increase co-stimulatory molecules on recipient APCs, leading to increased activation of donor T-cells and increased aGVHD [16].
In this study, the overall survival was significantly higher in low transfusion group (46.7%) than that in high transfusion group (25%) during the first year post-transplant. Alessandrino EP et al., also found that there was an inverse correlation between transfusion burden and overall survival after transplantation (p=0.022). Furthermore, they documented a positive correlation between transfusion burden and non-relapse mortality (p=0.024) and the outcome of transplanted patients, who received more than 20 red cell units were significantly worse [17]. In another study Hosoba S et al., demonstrated that increased numbers of transfused RBC units (HR per 2 units =1.17, p<0.0001) was also associated with worse long-term survival in univariable analysis and they attributed this results to demonstrate significant association of RBC transfusions with the subsequent development of grade III-IV aGVHD [18].
Limitation
This study had a number of limitations related to its retrospective nature and lack of serum ferritin data for the transplanted patients. Second, because the included patients had different types of haematological malignancies and received different pre-transplant conditioning regimen which would affect their transfusion requirements, disease free survival and overall survival, however if the patients were divided into many subgroups according to the primary disease, that would have affected the statistical power of the study.
Conclusion
The data presented in the current study has highlighted the adverse impact of high transfusion burden on the AHSCT outcome as regard to the overall survival and post-transplant complications. Thus, new rationale for more restricted transfusion practice based on symptoms driven criteria for these patients is highly warranted.
Non: Patients with no transfusion requirements; PLTS: Platelets transfusion; RBCs: packed Red blood cells transfusion