Introduction
The prevalence of Chronic Kidney Disease (CKD) in Iran amounts to 15.14%, which is higher than the global average. Given the substantial cost of this disease, health insurance companies need evidence of the cost-effectiveness of screening for the disease in adults at risk so that they can control the prevalence of the disease and the associated incremental medical costs by implementing a nationwide screening.
Aim
To explore the cost-effectiveness of screening for CKD among adults as compared with having a non-intervention strategy.
Materials and Methods
The study had a cross-sectional design and uses the cost-effectiveness analysis approach to compare the costs and outcomes of screening versus non-screening CKD. The estimated Glomerular Filtration Rate (GFR) was used for primary screening of the population. For this purpose, blood creatinine was measured; subsequently, urine creatinine and volume were analysed. However, some complementary measures and diagnostic tests were employed for positive cases, including the kidney ultrasonography. Costs and outcomes of the two strategies was calculated using a Markov decision model. This model is designed based on the natural course of CKD and GFR as a five-stage model. Costs of services required for patients were calculated based on Iran Health Insurance Organisation database, and outcome data were extracted in terms of the Quality-Adjusted Life-Year (QALY) index. Using TreeAge software, costs and outcomes were simulated for 1000 patients, and sensitivity analysis was used to test the reliability of the model data.
Results
For an adult population, the cost-effectiveness ratio for screening versus non-screening was 277,686,954 Rials per QALY, which was the effective cost. The results of one-way sensitivity analysis on the variables of the model shows that the screening strategy can be considered a dominant strategy in different domains.
Conclusion
Given the high prevalence of CKD in Iran, early detection of this disease via adult population screening is cost-effective for health insurance companies, and these organisations can control the costs of dialysis and kidney transplantation by reducing the rate of patient transitions from early to the final stages of the disease.
Introduction
CKD is a global public health concern. With an average annual growth rate of 8%, it holds one of the fastest rates among chronic diseases and has accordingly been considered by policy-makers as one of the public health challenges on the global scale [1-3]. The prevalence of this disease in developing countries overrides that of the developed ones. The average prevalence of this disease is reported to be 13.4 per 100 people worldwide [4].
To design the Markov model of the natural course of CKD, the different stages of the disease needs to be identified. For this purpose, five phases were identified using the Glomerular Filtration Rate (GFR) as recommended by the disease guideline [5,6].
As the patient moves from one stage to another stage, his/her quality of life decreases, and the burden of medical care increases due to the patients’ need for dialysis or transplantation as well as cardiac complications. Policy makers are seeking to prevent and delay patient transition to advanced stages of the disease where the patient may endure impaired kidney function [7,8].
CKD can have a considerable financial burden on families, the country, and insurance organisations because of dialysis, hospitalisation, and transplantation costs [9,10]. In 2015, Medicare Insurance in the United States spent over 98 billion USD on kidney failure patients [11]. In Canada, the cost is estimated at $ 260 million for 2017 [12]. In Australia, studies show that the annual cost of treatment for these patients will reach $ 1.1 billion by 2020 [13]. The huge cost of treatment and the lengthy treatment process have caused some patients to suffer catastrophic expenditure, putting them on the borders of poverty [4]. While kidney disease accounts for 1.1% of the global burden of disease [14], these circumstances have caused 35.6% of the patients in middle-to high-income countries to be exposed with the dreadful costs of the disease [10].
The predictions as per the increased incidence of CKD and the limitations associated with current treatments in eliminating kidney inefficiencies suggest the need for clinical and population-based interventions to prevent CKD [3]. The early detection of this disease via screening is one of the programs used to prevent or delay the effect of the disease, especially in high-risk groups such as those with high blood pressure and diabetes [7].
Considering the financial burden of implementing a large-scale screening at the community level, and the need to ensure the effectiveness of screening results, the use of cost-effectiveness analysis is essential for the economic evaluation of laboratory tests [15].
Numerous studies have been conducted in different countries to economically evaluate CKD screening, with differences in target groups, perspectives of analysis, and expected outcomes. The target population has mainly comprised of adults, senior citizens, and children [16-18]. The perspectives have included the community, service providers, the Ministry of Health, and funding providers to identify costs [19-21]. Concerning the analysis outcome, the studies have utilised the QALY index, the gained year of life, and the number of patients with end-stage prevention for the modeling process [22,23].
CKD has a prevalence rate of 15.14% in Iran, overriding that of the global average [24]. The lack of evidence on assessing the cost-effectiveness of screening for this disease underlies the motive to conduct this study. Moreover, analysis and modeling were made from the perspective of the insurance organisations/companies. This study was designed to provide some evidence for the policy-making of CKD screening among the adult population of Iran. The study evaluated the cost-effectiveness of screening versus non-screening CKD from the perspective of insurance organisations in Iran. The findings are expected to help policy makers to design interventions that can delay the disease progression to advanced stages and improve health outcomes.
Materials and Methods
This study was built on a cross-sectional design and the cost-effectiveness analysis method to compare CKD laboratory screening in terms of the GFR level [7] versus non-screening in the adult population of the Tehran province, Iran, from February 2016 to December 2017. This study was conducted as a computer simulation, and therefore no patient was studied in the field; hence, no need to formulate inclusion and exclusion criteria, informed consent, or ethical clearance approval. An analysis was made from the perspective of social insurance organisations. A discount rate of 5% was considered for all the benefits and costs. All the past costs were calculated based on the inflation rate of service tariffs covered by insurance companies. The cost-related data for the services provided for patients in line with the disease progression were extracted from the Health Insurer’s database. The final results were presented based on the Incremental Cost Effectiveness Ratio (ICER). The ratio is an indicator that illustrates the difference in cost between the strategy of using screening and the strategy of not using screening, divided by the difference in their outcomes. The level of effectiveness was considered as per the World Health Organisation criteria. It is a threshold used to indicate the cost-effectiveness of the estimated costs for one QALY [15]. That is, if the costs exceeded the average or were less than three times average per capita gross domestic product, they were considered cost-effective. This study built on one-way sensitivity analysis to increase and decrease the parameters of the model by 50% of their original value. The natural course of CKD was considered based on GFR and its five stages. The screening process is based on the GFR, whose initial calculation is by measuring blood creatinine, followed by an analysis of urine creatinine and volume. If the results confirm CKD, the kidney ultrasound is requested. [Table/Fig-1] shows the stages of the disease according to GFR.
Stages of CKD based on GFR.
| Stage | Pathology | GFR amount (mL/min/1.73 m2) |
|---|
| I | Kidney damage with normal GFR | ≥90 |
| II | Kidney damage with mild GFR | 60-89 |
| III | Moderately reduced GFR | 30-59 |
| IV | Severely reduced GFR | 15-29 |
| V | Kidney failure | <15 |
Using a Markov decision method [25,26], the study developed the model of the disease transition stages [5] in two scenarios, i.e., screening and non-screening, as presented in [Table/Fig-2]. Based on this model, the decision tree was built using the decision analysis software (Tree Age Pro 2015, Williamstown, MA). A 1000-people cohort was simulated via this computer model.
A Markov model of the natural course of the disease in screening and non-screening states.
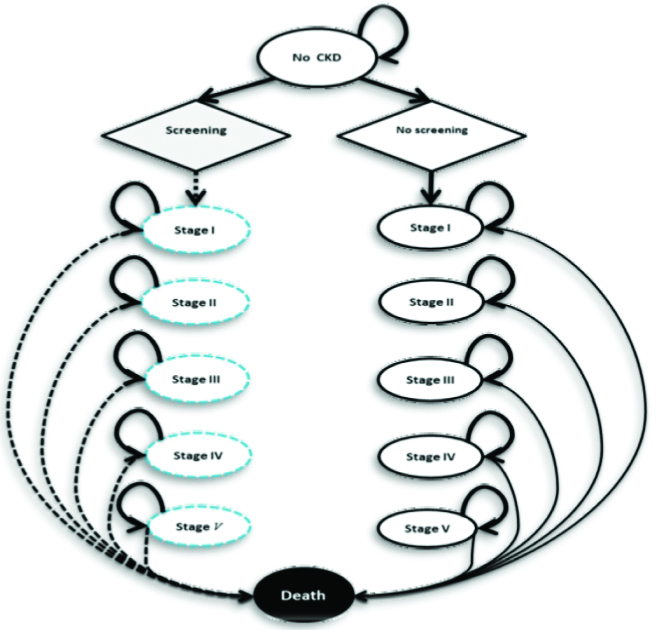
The stages of the disease and transition processes for the screening strategy are shown in dots.
Transition from the final stage of the disease to the cardiac disease stage resulting from CKD is not included in the model.
The initial distribution of CKD patients into different stages was based on the prevalence data in Iran. The transition probabilities between the five stages of the disease and the disease-associated death in the two strategies were derived from the literature [27]. They were finalised using the data from the patients’ electronic records file and after the panel between nephrologists and health economics experts was held [Table/Fig-3].
Transition probabilities between different stages of CKD in screening and non-screening states.
| Screening strategy | No screening strategy |
|---|
| No CKD | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | | No CKD | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 |
|---|
| No CKD | 0.874 | 0.022 | 0.021 | 0.078 | 0.003 | 0.002 | No CKD | 0.874 | 0.022 | 0.021 | 0.078 | 0.003 | 0.002 |
| Stage 1 | 0 | 0.27 | 0.62 | 0 | 0 | 0 | Stage 1 | 0 | 0.70 | 0.29 | 0 | 0 | 0 |
| Stage 2 | 0 | 0 | 0.34 | 0.46 | 0 | 0 | Stage 2 | 0 | 0 | 0.405 | 0.58 | 0 | 0 |
| Stage 3 | 0 | 0 | 0 | 0.37 | 0.27 | 0 | Stage 3 | 0 | 0 | 0 | 0.35 | 0.6475 | 0 |
| Stage 4 | 0 | 0 | 0 | 0 | 0 | 0.28 | Stage 4 | 0 | 0 | 0 | 0 | 0 | 0.24 |
| Stage 5 | 0 | 0 | 0 | 0 | 0 | 0.15 | Stage 5 | 0 | 0 | 0 | 0 | 0 | 0.80 |
Given the natural course of the disease [6] and the probability table, screening does not prevent the natural course or return to a previous stage of CKD, but it only reduces the risks of transition from an early stage of the disease to advanced stages, so that the final stages may occur at a delayed time. In this study, each stage was considered to last for a year. The study did not cover CKD complications, e.g., cardiac complications, in terms of the transition probabilities and economic burden, nor did it include the probability of death occurring as a result of other causes.
Estimates of costs were evaluated from the perspective of insurance organisations. Costing was performed as aggregating parts to a whole using the questionnaire of services prescribed at each stage of the disease using experts’ opinions. Therefore, one questionnaire was developed for each CKD stage, which identified the type and frequency of the prescribed services as well as the proportion of prescription for the patient population at each stage. The services included laboratory tests, diagnostic imaging, medication, dialysis, and transplantation. The questionnaire was completed by a panel of nephrologists and was finalised after unanimous agreement was reached by them. The cost of services was calculated using the health insurance organisation’s database according to the tariffs approved by and the payment made by the organisation.
Values of desirability were calculated in terms of QALY. QALY’s value for each stage of the disease was extracted based on the literature [27] and is shown in [Table/Fig-4].
Desirability of different states of model.
| State | Well | Stage I | Stage II | Stage III | Stage IV | Stage V |
|---|
| Utility | 1 | 0.96 | 0.92 | 0.88 | 0.84 | 0.80 |
Results
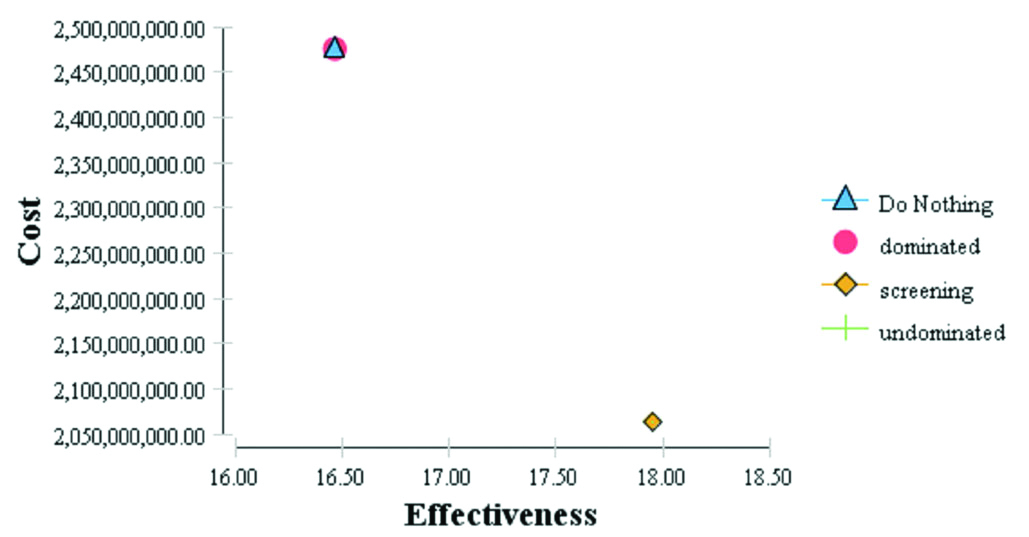
As shown in [Table/Fig-5], the ratio of the compared cost-effectiveness was calculated for both the screening and non-screening strategies.
Results of differential cost-effectiveness analysis of screening versus non-screening.
| Subset | Strategy | Effectiveness | Incremental effectiveness | Cost | Incremental cost | Incremental Cost/ Incremental effectiveness |
|---|
| Dominated | No screening | 16.46 | -1.48 | 2,475,492,589 | 412,568,667 | - 277,686,954 |
| Undominated | Screening | 17.95 | 0 | 2,062,923,922 | 0 | 0 |
The results of the ICER analysis showed that the screening strategy can be considered a superior strategy. This strategy generates more output (QALY). Interpreting this ratio shows that the cost of a QALY unit (one year of full health) has an extra cost through a screening as much as 277,686,954 Rials. [Table/Fig-6] shows the results of this analysis.
A graph of the cost-effectiveness difference.
Sensitivity Analysis
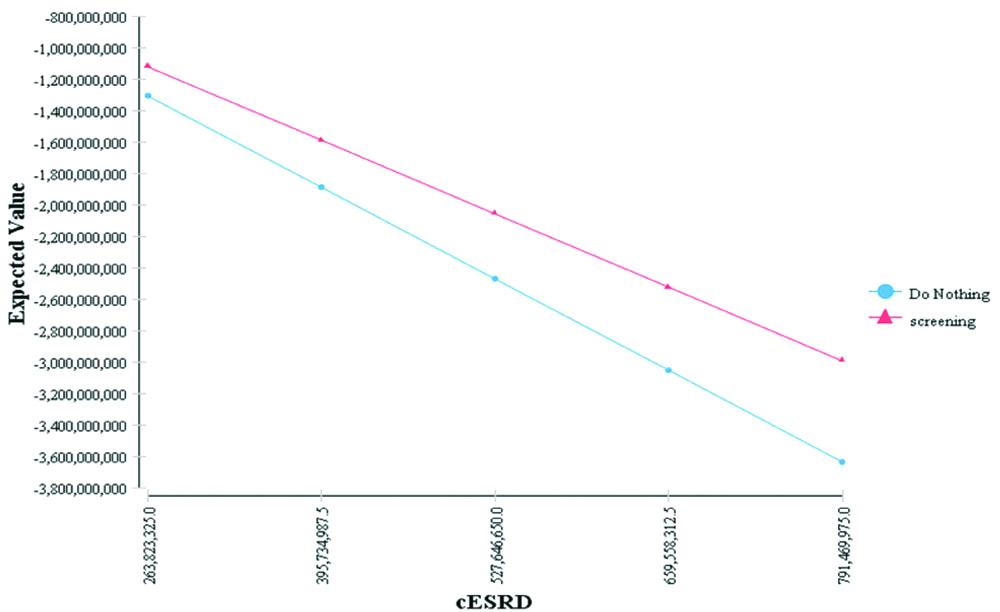
Regards to the greater weight of end-stage costs variable of CKD, the one-way sensitivity analysis was performed for mentioned variable. Considering that during the data collection, sharp currency fluctuations were dominant in Iranian economy and due to coping with the methodological errors, the domain of the variables was considered to be 50±%. The results of sensitivity analysis on the indicated variable showed that screening strategy was considered as a dominant strategy in different domains [Table/Fig-7].
One-way sensitivity analysis of end-stage costs variable of CKD.
Discussion
Given the increasing rate of CKD incidence, the use of strategies for the early detection of the disease and the control of its progression, especially in the adult population, can both improve the quality of life of patients and reduce the health costs of the disease for insurance organisations.
As indicated in the present study, adult population screening is cost-effective based on the calculation of GFR. Some studies have indicated screening to be ineffective, such as Manns B et al., study in Canada similar to present study from a financial provider’s perspective, assessing the economic screening of the disease, whose result is ineffectiveness of screening for the entire population and even specific groups such as hypertension or the elderly [17].
The studies conducted by Sekhar DL et al., in the United States have shown that spending money on laboratory screening to diagnose CKD in the pediatric population is not cost-effective [16]. Some authors suggest that only high-risk individuals should be considered as target groups such as the elderly or people with the hypertension or diabetes, like Craig JC et al., study in Australia, which has been done with considering the number of preventable cases from the final stage of kidney disease [22], as well as den Hartog JR et al., study conducted in the United States using the QALY analysis [23]. The two studies considering the provider’s perspective for delivering the service for analysing, screening of older people have been recommended [22,23]. Hoerger TJ et al., study in the United States recommends screening for people with diabetes and high blood pressure, and considers screening beneficial for the whole population, if it is done in long intervals, or considered as part of the package of current care services for doctors [18].
Studies in Australia, the Netherlands and Japan have evaluated screening for the entire adult population as an effective cost. These studies have been conducted from a wider perspective of the community, financial supplier, and provider of health services and the outcomes are based on QALY or life years gained [19-21,28].
One of the most important factors in these studies seems to be the prevalence rate of the disease in the target population. In Asian countries such as Iran, whose prevalence is above average, it is advisable to conduct screening of the entire population, but in countries such as Europe and the United States that the prevalence is less, policy makers are advised to focus on specific populations that have the risk of suffering from the disease to ensure about consuming optimal resources.
Limitation
- Since the perspective of the present study is from the eyes of insurance companies, the cost of payment by patients and their families is not included in the calculations. Also, the costs imposed to the community that are related to the changes in the productivity of people with CKD are not considered, and so the cost section of the model may be less estimated.
- Using the simulation model due to lack of data access.
- Not considering the stage of heart disease caused by CKD and its resulted death.
- Not counting on the survival table.
Conclusion
Given the increasing trend of CKD, which results in the growth of demand for costly dialysis and kidney transplant services, insurance organisations must prioritise the prevention and control of the disease in their early stages, and through screening the population of insured persons undertake to identify patients and support their treatment. This will keep patients at an early stage of the disease and prevent the development of severe impairment in kidney function and also the incidence of dialysis and transplantation costs. By doing so, they will be more healthy and have a higher quality of life for covered insured persons. It will help manage the cost of treatment and spend on the expansion of the covered population under the resources saved and increasing the service package, and by reducing the cost of paying money from the pocket, prevent its effects on poverty creation.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? NA
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 16, 2019
Manual Googling: Oct 01, 2019
iThenticate Software: Oct 23, 2019 (5%)
[1]. El Nahas AM, Bello AK, Chronic kidney disease: The global challengeThe Lancet 2005 365(9456):331-40.10.1111/j.1523-1755.2005.00774.x16316385 [Google Scholar] [CrossRef] [PubMed]
[2]. Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Global prevalence of chronic kidney disease- A systematic review and meta-analysisPLoS One 2016 11(7):e0158765eCollection 201610.1371/journal.pone.015876527383068 [Google Scholar] [CrossRef] [PubMed]
[3]. Alebiosu CO, Ayodele OE, The global burden of chronic kidney disease and the way forwardEthn Dis 2005 15(3):418-23. [Google Scholar]
[4]. Nugent RA, Fathima SF, Feigl AB, Chyung D, The burden of chronic kidney disease on developing nations: A 21st century challenge in global healthNephron Clin Pract 2011 118(3):c269-77.Epub 2011 Jan 710.1159/00032138221212690 [Google Scholar] [CrossRef] [PubMed]
[5]. Halpin David SP, Chronic kidney disease, National clinical guideline for early identification and management in adults in primary and secondary careThe National Collaborating Centre for Chronic Conditions 2008 [Google Scholar]
[6]. Nelson Robert G. TKR, Clinical Practice Guidelines and Clinical Practice Recommendations for diabetes and chronic kidney diseaseOfficial journal of the National Kidney Foundation 2007 49(2)10.1053/j.ajkd.2006.12.005 [Google Scholar] [CrossRef]
[7]. Levey Andrew S, CJ. Clinical practice guidelines for chronic kidney disease: Evaluation, Classification and StratificationNational Kidney Foundation 2002 [Google Scholar]
[8]. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY, Chronic kidney disease and the risks of death, cardiovascular events, and hospitalizationN Engl J Med 2004 351(13):1296-305.10.1056/NEJMoa04103115385656 [Google Scholar] [CrossRef] [PubMed]
[9]. Luyckx VA, Tonelli M, Stanifer JW, The global burden of kidney disease and the sustainable development goalsBull World Health Organ 2018 96(6):414-22D.Epub 2018 Apr 2010.2471/BLT.17.20644129904224 [Google Scholar] [CrossRef] [PubMed]
[10]. Essue BM, Laba M, Knaul F, Chu A, Minh HV, Nguyen TKP, Economic burden of chronic ill health and injuries for households in low- and middle-income countries In: Jamison D T, Gelband H, Horton S, Jha P, Laxminarayan R, Mock C, et alDisease Control Priorities: Improving Health and Reducing Poverty 2018 WashingtonThe World Bank:121-46.10.1596/978-1-4648-0527-1_ch6 [Google Scholar] [CrossRef]
[11]. System USRD, 2015 USRDS annual data report: Epidemiology of Kidney Disease in the United StatesNational Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 2015 [Google Scholar]
[12]. Manns B, McKenzie SQ, Au F, Gignac PM, Geller LI, The financial impact of advanced kidney disease on canada pension plan and private disability insurance costsCan J Kidney Health Dis 2017 4:2054358117703986eCollection 201710.1177/205435811770398628491340 [Google Scholar] [CrossRef] [PubMed]
[13]. Cass A CS, Gallagher M, Howard K, Jones A, McDonald S, Snelling P, White S, The economic impact of end-stage kidney disease in Australia 2010 [Google Scholar]
[14]. World Health Organization, Global Health Estimates 2014. Geneva, Switzerland; 2014. Available from:http://www.who.int/healthinfo/global_burden_disease/en/. Last accessed on 10 Jul, 2019 [Google Scholar]
[15]. Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B, Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge?Value Health 2004 7(5):518-28.10.1111/j.1524-4733.2004.75003.x15367247 [Google Scholar] [CrossRef] [PubMed]
[16]. Sekhar DL, Wang L, Hollenbeak CS, Widome MD, Paul IM, A cost-effectiveness analysis of screening urine dipsticks in well-child carePediatrics 2010 125(4):660-63.Epub 2010 Mar 1510.1542/peds.2009-198020231188 [Google Scholar] [CrossRef] [PubMed]
[17]. Manns B, Hemmelgarn B, Tonelli M, Au F, Chiasson TC, Dong J, Population based screening for chronic kidney disease: Cost effectiveness studyBMJ 2010 341:c5869-c.10.1136/bmj.c586921059726 [Google Scholar] [CrossRef] [PubMed]
[18]. Hoerger TJ, Wittenborn JS, Segel JE, Burrows NR, Imai K, Eggers P, A health policy model of CKD: 2. The cost-effectiveness of microalbuminuria screeningAm J Kidney Dis 2010 55(3):463-73.Epub 10 Feb 810.1053/j.ajkd.2009.11.01720116910 [Google Scholar] [CrossRef] [PubMed]
[19]. Atthobari J, Asselbergs FW, Boersma C, de Vries R, Hillege HL, van Gilst WH, Cost-effectiveness of screening for albuminuria with subsequent fosinopril treatment to prevent cardiovascular events: A pharmacoeconomic analysis linked to the Prevention of renal and vascular endstage disease (PREVEND) study and the Prevention of renal and vascular endstage disease intervention trial (PREVEND IT)Clin Ther 2006 28(3):432-44.10.1016/j.clinthera.2006.03.01216750458 [Google Scholar] [CrossRef] [PubMed]
[20]. Boersma C, Gansevoort RT, Pechlivanoglou P, Visser ST, van Toly FF, de Jong-van den Berg LT, Screen-and-treat strategies for albuminuria to prevent cardiovascular and renal disease: cost-effectiveness of nationwide and targeted interventions based on analysis of cohort data from the NetherlandsClin Ther 2010 32(6):1103-21.doi: 10.016/j.clinthera.2010.06.01310.1016/j.clinthera.2010.06.01320637965 [Google Scholar] [CrossRef] [PubMed]
[21]. Kondo M, Yamagata K, Hoshi SL, Saito C, Asahi K, Moriyama T, Cost-effectiveness of chronic kidney disease mass screening test in JapanClin Exp Nephrol 2012 16(2):279-91.Epub 2011 Dec 1410.1007/s10157-011-0567-122167460 [Google Scholar] [CrossRef] [PubMed]
[22]. Craig JC, Barratt A, Cumming R, Irwig L, Salkeld G, Feasibility study of the early detection and treatment of renal disease by mass screeningIntern Med J 2002 32(1-2):6-14.10.1046/j.1445-5994.2002.d01-11.x11783681 [Google Scholar] [CrossRef] [PubMed]
[23]. den Hartog JR, Reese PP, Cizman B, Feldman HI, The costs and benefits of automatic estimated glomerular filtration rate reportingClin J Am Soc Nephrol 2009 4(2):419-27.Epub 2009 Jan 2810.2215/CJN.0408080819176794 [Google Scholar] [CrossRef] [PubMed]
[24]. Bouya S, Balouchi A, Rafiemanesh H, Hesaraki M, Prevalence of chronic kidney disease in Iranian general population: A meta-analysis and systematic reviewTher Apher Dial 2018 22(6):594-99.Epub 2018 Jul 410.1111/1744-9987.1271629974630 [Google Scholar] [CrossRef] [PubMed]
[25]. Sonnenberg FA, Beck JR, Markov models in medical decision making: a practical guideMed Decis Making 1993 13(4):322-38.doi: 10.1177/0272989X930130040910.1177/0272989X93013004098246705 [Google Scholar] [PubMed] [PubMed]
[26]. Grewal JK, Krzywinski M, Altman N, Markov models-Markov chainsNat Methods 2019 16(8):663-64.10.1038/s41592-019-0476-x31363204 [Google Scholar] [CrossRef] [PubMed]
[27]. Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysisHealth Technol Assess 2010 14(21):1-184.10.3310/hta1421020441712 [Google Scholar] [CrossRef] [PubMed]
[28]. Howard K, White S, Salkeld G, McDonald S, Craig JC, Chadban S, Cost-effectiveness of screening and optimal management for diabetes, hypertension, and chronic kidney disease: A modeled analysisValue Health 2010 13(2):196-208.Epub 2009 Oct 2910.1111/j.1524-4733.2009.00668.x [Google Scholar] [CrossRef]