Among orthopaedic pathologies in childhood, spinal curvature is most common one in children of 10-18 years age group. IS has a frequency of up to 2-3% in the population. However, the nature of the spinal deformity in patients with IS is different: in some, the curvature has a rapid progression rate, in others-the magnitude of the deformity remains almost unchanged until the end of growth [1].
According to the literature, several groups of factors that have the most value and affect the nature of the pathological process in IS can be distinguished: anatomical and anthropometric parameters of spinal deformity (torsion value of the apical vertebra, Cobb angle value, axial vertebral rotation, etc.,) [2]; genetic hereditary background, various mutations of genes [3-5]; characteristic of the surrounding tissues state [6]; endocrine system and hormonal disorders [7]; associated anomalies of the spinal canal and spinal cord [8,9].
From the view of determining the tactics of treating a patient with IS, it is very important to timely assess the nature of the spinal deformity course in the early stages of the deformity development and select a group of patients with a progressive nature of the disease [10]. This is necessary for the implementation of adequate therapy to correct the curvature and prevent further development of the curvature.
Identification of the etiological factors of IS is impossible without in-depth molecular genetic studies that allow to identify the biological basis of the pathological process. To date, many studies have been carried out, highlighting this problem from different angles [11]. Various groups of researchers performed work on the analysis of connective tissue genes, such as Type 1 collagen A1 and A2 (COL1A1, COL1A2), Type 2 collagen A1 (COL2A1), Elastin (ELN), Aggrecan (ACAN), Fibrillin (FBN1) and others. However, the results of these studies did not show a significant relationship between the different polymorphisms studied and the rate of progression of IS [12-14].
Although the folate cycle genes are almost unexplored in patients with IS, these genes are responsible for the activity of enzymes in methylation reactions, which are responsible for many enzyme transformations, including melatonin metabolism. Given the fact that the deficiency and anomalies of the melatonin signal system are one of the aetiological factors of IS [15,16], it can be assumed that changes in the genes responsible for encoding the structure and activity of folate cycle enzymes may be associated with IS [17].
The Methylenetetrahydrofolate Reductase (MTHFR), Methionine synthase (MTR), Methionine Synthase Reductase (MTRR) genes are the most studied among the folate cycle genes. It is the activity of the enzymes encoded by these genes that determines the quantity and quality of the final products of folate metabolism [18,19].
The IL-6 gene encodes IL-6, which can act as a proinflammatory and anti-inflammatory cytokine depending on the specific situation [20,21]. Cytokines like IL-6 play important role in bone homeostasis, stimulating the development of osteoclasts. There is more evidence that these cytokines contribute to the development of osteoblasts [22]. Gene IL-6 can be considered susceptible to changes in the IS coefficient. Identification of molecular markers with diagnostic and predictive purposes may be useful for early detection of children with a group risk for the development of scoliosis and to predict the risk of rapid progression of deformity [23,24].
The previous similar study was done by the same authors, on the folate group genes, on a small sample of patients with IS and the control group (48 and 32 people, respectively) [25]. This allowed the authors to identify some patterns but did not give adequate statistical reliability of the results. The number of examined patients and the number of the control group have increased. In addition, the authors continue to search for new genetic markers for IS and performed the study of the IL-6 G(-174)C gene polymorphism. The aim of this study was to analyse molecular genetic profile of folate cycle genes and IL-6 gene polymorphism in children with progressive type of IS.
Materials and Methods
Participants
This case-control study of folate cycle genes was done on 110 children with progressive type of IS of third and fourth stage (curves more 25°) in the age group from 14 years to 18-years with complete bone growth (Risser sign [26]-Grade 4-5). These patients underwent treatment at the Turner scientific research Institute for children’s orthopaedics, St-Petersburg, Russia from September 2016 to April 2019.
Among the survey there were 22 boys and 88 girls. The Cobb angle ranged from 38° to 146°, on average-92°. The study group did not include children under the age of 14 and over 18, as well as with the developmental defects of the spinal cord and spinal canal. In the clinical picture, neurological disorders were not observed in any child.
According to the localisation of the main scoliotic arcs, the study included patients with thoracic (70 children), lumbar (7), thoracolumbar (9) and combined (24) IS.
The control group consisted of 110 healthy volunteers who did not have orthopaedic pathology and spinal deformity with complete bone growth (Risser sign-4-5 points).
This study was performed according to the Declaration of Helsinki after being approved by the Local Ethics Committee of the Institute. Parents of the all underage participants were provided an informed consent for their participation in the study.
Genomic DNA Extraction and SNP Selection
Genomic DNA was extracted from 2 mL whole blood samples using a genomic DNA extraction kit (Inter Lab Service Ltd., Russia) following the manufacturer’s protocols.
The polymorphic variants of folate metabolism genes were investigated: MTHFR A1298C (rs 1801131), MTHFRC677T (rs 1801133), MTRA2756G (rs 1805087), MTRRA66G (rs 1801394).
The polymorphism of the IL-6G(-174)C(rs1800795) gene was also analysed.
Analysis of genetic polymorphisms of folate genes was performed using Real-time PCR method with SNP-Screen kits (Sintol, Russia) on the CFX96 Touch™ Real-Time PCR Detection System Analyser (Bio-Rad Laboratories, USA).
Analysis of the IL-6 gene polymorphism G(-174)C was performed using the basic PCR method with electrophoretic detection. To identify the G(−174)C polymorphism, a 198 bp fragment was amplified using the forward primer 5′-TGACTTCAGCTTTACTCTTTGT-3′ and the reverse primer 5′-CTGATTGGAAACCTTATTAAG-3′; the conditions of the reaction were as follows: denaturation at 95°C for 3 minutes, followed by 35 cycles of annealing at 55°C for 60 seconds, extension at 72°C for 60 seconds, and a final elongation step extension at 72°C for 5 minutes [23]. To determine the nucleotide substitutions, the Restriction fragment length polymorphism (RFLP) was used. Restriction of the amplified DNA fragments was performed using restriction endonuclease SfaN1 (SibEnzyme Ltd., Russia) according to the manufacturer’s instructions. The results of PCR and RFLP were analysed by DNA gel electrophoresis in 9% polyacrylamide gel followed by colouring in SYBR Green I (Biotech Industry Ltd., Russia) and visualisation of fragments in the UV light [Table/Fig-1].
Electrophoresis of amplified SNP IL-6 (-174G/C). Lanes are respectively: ladder: Ladder 100 bp; 1: C174C; 2: G174G: 3: C174C; 4: G174C; 5,6: C174C: 7: G174G.
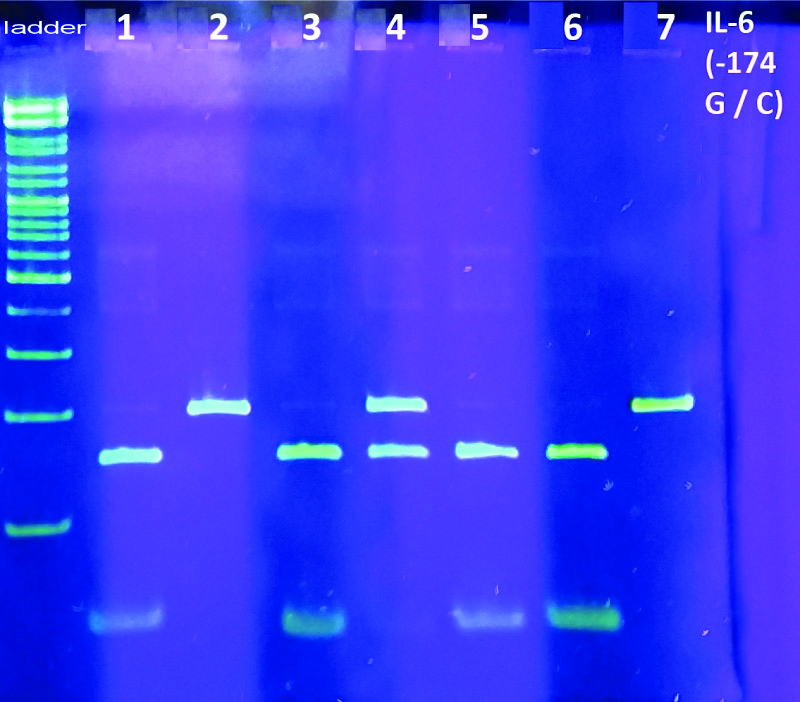
The unrestricted 198 bp fragment corresponded to the G174G genotype, restrictive 198/140/58 bp fragments corresponded to the heterozygous genotype G174C, and the restricted 140/58 bp fragments corresponded to the C174C genotype.
Statistical Analysis
Results were statistically processed using the software package Statistica 6.0. Group comparisons with respect to categorical variables were performed using chi-square tests or, alternatively, Fisher’s-exact test when expected cell counts were less than 5. A p-value less than 0.05 were considered significant.
Results
A total of 110 children with progressive form of IS were in the age group from 14 to 18-year-old. Among the survey were 22 boys and 88 girls. The Cobb angle ranged from 38 to 146°, on average-92°.
According to the localisation of the main scoliotic arcs, the study included patients with thoracic (70 children), lumbar (7), thoracolumbar (9) and combined (24) IS.
It was not possible to collect a family history of patients, as many patients were from religious families and their parents were not inclined to give interviews.
The control group consisted of 110 people without spinal pathology aged 15 to 32 years were studied for the polymorphism of the folate cycle genes and the IL-6 gene.
Genotype distribution results in IS children group and control group are presented in [Table/Fig-2,3,4,5,6 and 7].
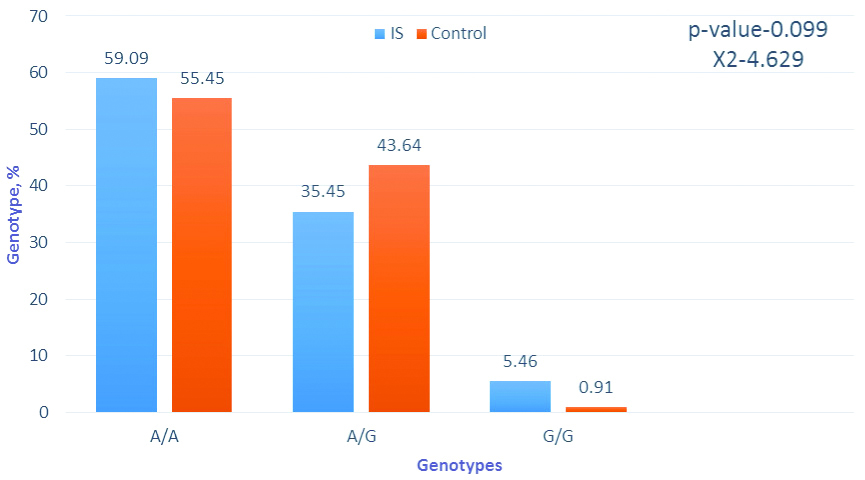
Frequency of genotypes by the gene MTHFR A1298C, % software package statistica 6.0 and chi-square tests were applied for analysis.
Frequency of genotypes by the gene MTHFR C677T, % software package statistica 6.0 and chi-square tests were applied for analysis.
Frequency of genotypes by the gene MTRA2756G. Software package statistica 6.0 and chi-square tests were applied for analysis.
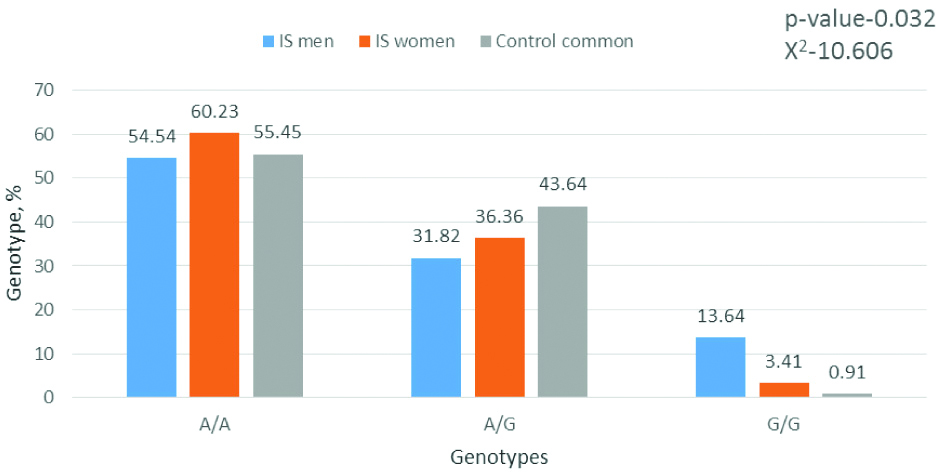
Frequency of genotypes by the gene MTRA2756G (is men, is women, and control group common). Software package statistica 6.0 and chi-square tests were applied for analysis.
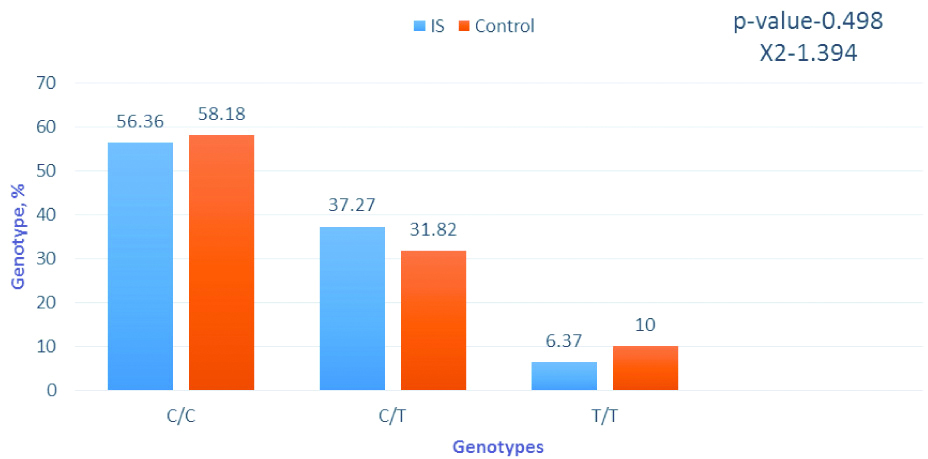
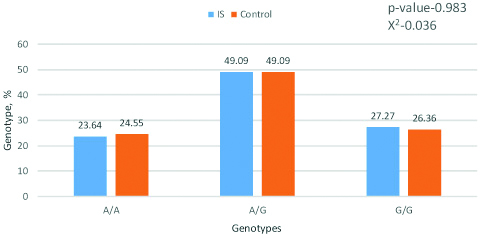
Frequency of genotypes by the gene MTRR A66G. Software package statistica 6.0 and chi-square tests were applied for analysis.
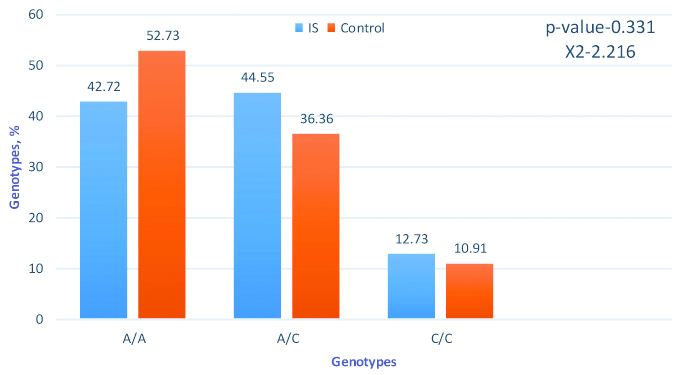
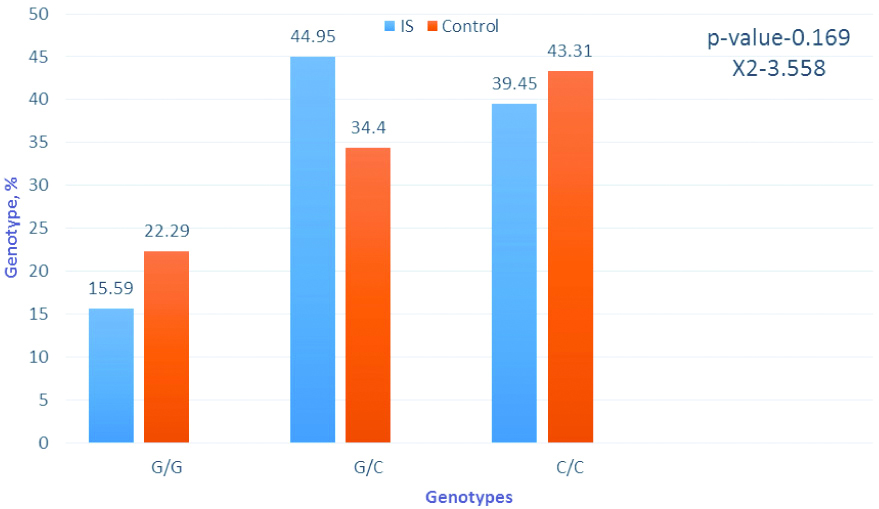
Frequency of genotypes by the gene IL-6 G (-174)C. Software package statistica 6.0 and chi-square tests were applied for analysis.
The distribution of the A1298C alleles polymorphism shows that A1298A homozygotes for the major allele in IS patients are 1.23 times less common than in the control, while the aggregate of patients with an adverse minor allele 1298C is 57.28%, while in the control this indicator is only 47.27%. The frequency of A-alleles in IS patients was 65% and C-alleles-35%, while in the control group this ratio was 70.91% and 29.09%.
The reverse pattern was observed for the C677T polymorphism of the MTHFR gene. The adverse allele in the control group was even more frequent, the differences between the groups are very small, and this suggests that, clearly, the definition of the C677T polymorphism of the MTHFR gene in patients with IS has no clinical and diagnostic value.
The distribution of genotypes and alleles of the A2756G polymorphism showed an interesting feature: while the number of patients with IS with an unfavourable genotype of 2756G was 40.91%, and in the control group this value was even slightly higher (44.55%), homozygous carriers of the unfavourable allele 2756G met 6 times more often in patients with IS. It was also investigated whether there were gender differences in the distribution of genotypes in IS between men and women, especially since IS are more common among women than among men. The gender distribution of genotypes were analysed by the studied polymorphisms and found that there were significant differences in the MTR A2756G polymorphism [Table/Fig-5].
The results of the genotypes and alleles distribution analysis in the MTRR A66G polymorphism did not show significant differences between patients with IS and the control group. Obviously, this polymorphism has no effect on the development of the disease.
The study of the G(-174)C polymorphism of the IL-6 gene revealed some differences between patients with IS and the control group [Table/Fig-7].
An interesting feature was also found in the distribution of genotypes and alleles of the IL-6G (-174) C polymorphism: carriers of the unfavourable allele in the group of patients with IC were 84.4%, while in the control group this indicator was 77.71%. At the same time, homozygous carriers of the unfavourable allele in the control group were slightly higher (by 3.86%).
Discussion
In the present study, author investigated a group of children with progressive type of IS of third and fourth stage (curves more 25°) in the age group from 14 to 18-year-old with complete bone growth and with an indication for surgery. The optimal age at which deformity correcting operations will be justified and effectively begins at 13-15 years. Otherwise, due to the active growth of the patient’s bones, corrective fixation systems can lead to undesirable consequences. It was this group that was considered for research.
A study of the folate group and IL-6 genes polymorphism showed that there are some differences in the distribution of genotypes between groups with IS and the control group for individual mutation spots.
Currently, many papers have been published on the analysis of polymorphic folate cycle genes. The folate cycle in the human body is a complex cascade process, which is regulated by a large number of enzymes, the product of which requires the presence of folic acid and B vitamins. In this complex process, methyl groups are transferred, homocysteine is metabolised, an excess of which turns into an essential amino acid methionine. Methionine, in turn, turns into S-adenosylmethionine, which in the cell is the main donor of methyl groups necessary for the synthesis and methylation of DNA, RNA, proteins and phospholipids, as well as the synthesis of dopamine, norepinephrine, serotonin, glycine, guanidine acetate, hormones and et al [27]. Deficiency of folic acid and B vitamins, polymorphism in folate metabolism genes, causing a decrease in enzyme activity, lead to excessive accumulation of homocysteine In the blood and, as a consequence, disruption of methylation processes in cells, which becomes the basis for the development of such pathological conditions like atherosclerosis, atherothrombosis, defect of the neural tube, heart attacks, violation of chromosome discrepancy in oogenesis (increases the risk of Down syndrome) [28,29].
Sometimes this can be significant, as it turned out, in gender differences, especially since there is a significant difference in the incidence of IS between men and women.
IS is most likely a multifactorial disease, in which a number of genes influence the development of this pathology. The folate group and IL-6 genes to a certain extent contribute to the development of IS, but nonetheless are obviously not the only reason for the development of IS. Thus, folate group genes may be associated with the metabolism of tetrahydrobiopterin (BH4), which serves as a cofactor for aromatic amino acid hydroxylases [30]. These enzymes are necessary for the synthesis of neurotransmitters such as serotonin and dopamine. An imbalance of neurotransmitters according to the literature [31] was found in patients with adolescent IS, which was not found in the control group of the same age. Moreover, when the balance was eliminated by medical measures, these patients had a correction of scoliotic deformity compared with patients who were not treated in this way [32].
The IL-6 gene, on the other hand, is involved in a whole cascade of reactions associated with an increase in the level and activity of inflammatory cytokines, which can lead to degeneration of intervertebral disks. In addition, other metabolites associated with the inflammatory response, such as eicosanoids, are present in the degenerating tissues of the intervertebral disc. These agents enhance the expression of the matrix degrading enzymes like matrix metalloproteinase. The elevation in the activities of these enzymes promotes the breakdown of aggrecan, the major proteoglycan of the extracellular matrix in cartilaginous tissue and it withstands compression in cartilage. These agents cleave proteoglycans and fibrillar proteins of the disc of extracellular matrix also, the cytokines promoting important catabolic processes and regulate cell metabolism in a number of ways [33].
It has also been shown [34] intervertebral disc tissue was cultivated from patients undergoing surgery for IS, and then the level of anti-inflammatory cytokines was analysed. It was concluded that the scoliotic pulpal nucleus may respond to an exogenous pro-inflammatory stimulus with an increased amount of IL-6 and other cytokines.
The G/C polymorphism of the promoter region of the IL-6 gene affects the level and functional activity of the cytokine IL-6 [23]. The study of the role of IL-6 polymorphism G(-174)C in the pathogenesis of IS was conducted for the first time [35]. It was concluded that the polymorphism of IL-6 G(-174)C is an important factor in the genetic susceptibility to scoliosis. However, the distribution of genotypes in this study in patients with IS and control was different from that in the Bulgarian and Italian populations [23,35]. The distribution of genotypes revealed by author is characteristic of the Russian population and is confirmed in other work by Russian researchers who studied polymorphism G(-174)C [36,37].
It is possible that there are other genetically penetrant variants that occur simultaneously in patients with IS, and, thus, increasing the likelihood of developing spinal deformity [29]. In addition to genetic factors environmental factor may have an effect on patients with IS that do not affect non-scoliotics. Non-scoliotic patients may also not be exposed to the same environmental factors that contribute to the onset of IS [17].
This study conducted for the first time on the Russian population of patients with IS does not contradict previous studies that the mutations in the folate cycle and IL-6 genes can lead to progression of IS. Further studies will be carried out to identify the most significant polymorphisms of neurotransmitters genes that most significantly affect the violation of spine ontogenesis, causing the appearance of the disorders which leads to severe progressive spine deformations in IS.
Limitation
Because of the limited time, author couldn’t study the polymorphism of neurotransmitter and BH4 metabolism genes, which may play an important role in the aetiology and pathogenesis of IS.
Conclusion
The study of patients with IS made it possible to identify a part of the unfavourable genetic load contributing to the emergence and progression of this severe pathology. It was determined that children with IS have some differences in the distribution of genotypes between groups with IS and the control group for folate cycle and IL-6 genes polymorphisms.
The obtained results allow (to some extent) to assume the nature of the course of the spine deformities in patients with IS. However, a more detailed assessment and identification of the most significant molecular genetic criteria for the progression of the idiopathic spine deformities in children requires further extensive study.