Cytomegalovirus Seroprevalence and Immunity in Indian Perinatal Women: Experience of a Cord Blood Bank
Satyen Yash Sanghavi1, Tripti Upasso Gaunkar2, Vimal Vishvanath Gokale3, Vinayak Virupaksh Kedage4, Rucha Ponkshe5, Dinesh Vyas6
1 Chief Scientific Officer, Department of Stem Cell Technology, Acme Plaza, Mumbai, Maharashtra, India.
2 Medical Director, Department of Microbiology, Regrow Biosciences Private Limited, Lonavala, Maharashtra, India.
3 Quality Control Officer, Department of Microbiology, Regrow Biosciences Private Limited, Lonavala, Maharashtra, India.
4 Lab Director, Department of Microbiology, Regrow Biosciences Private Limited, Lonavala, Maharashtra, India.
5 Associate Vice President, Department of Clinical Research, Acme Plaza, Mumbai, Maharashtra, India.
6 Medical Director, Department of Pathology, Regrow Biosciences Private Limited, Lonavala, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vinayak Virupaksh Kedage, Regrow Biosciences Pvt. Ltd., 22, Shah Industrial Estate, Nangargaon, Lonavala-410401, Maharashtra, India.
E-mail: drvinayak@regrow.in
Introduction
Cytomegalovirus (CMV) is a DNA virus and member of herpes virus family, which is known to frequently infect, a growing fetus by vertical transmission. Since the frequency of congenital infection in newborn infants tends to vary with the prevalence of infection in population, the need to determine immunity against CMV, in perinatal women cannot be over emphasised.
Aim
To perform a retrospective data analysis over 8 years to find out seroprevalence of CMV in Indian women in perinatal period.
Materials and Methods
Large scale retrospective data analysis was carried out over eight years and on 23,029 maternal samples tested in perinatal period with uncomplicated pregnancies, at Regrow Biosciences Private Limited, India. The humoral response to CMV infection was determined by ELISA test for CMV IgG and IgM.
Results
Out of 23,029 maternal samples, 21,312 (92.5%) were IgG positive, 1,717 (7.5%) were CMV IgG negative and 23,029 (100%) were CMV IgM negative.
Conclusion
CMV infection is significant factor for Bad Obstetric History (BOH) and results in poor fetal outcome. Therefore, extensive serological screening of CMV infection attains utmost importance to know the factual status of this infection amongst women of childbearing age. Overall conclusion is that in Indian women population, zero prevalence of active CMV infection with uncomplicated pregnancy has been observed. However, the prevalence of high IgG antibody titers is substantial (92.5%) which is consistent with findings in other developing countries.
Sero-positivity, Uncomplicated pregnancy, Vertical transmission
Introduction
Human Cytomegalovirus (HCMV), formerly known as salivary gland viruses, is an omnipresent beta human herpes virus type 5 and is largest amongst all other human herpes virus with genomic mass of ~235 kb and encoding ~165 genes [1]. This virus is a linear double-stranded DNA virus with icosahedral nucleocapsid, enveloped by proteinaceous matrix, which is also known as tegument and it also exhibits strict host specificity [2]. CMV disease is rare but infection with the virus is very common. It spreads slowly but it requires close contact for transmission via saliva, other secretions and sexual contact. Other modes of transmission are transplacental, breastfeeding, blood transfusion, Solid-Organ Transplantation (SOT), or Hematopoietic Stem Cell Transplantation (HSCT) [3]. Like other herpes viruses, primary infection in immunocompetent children and adults is commonly inapparent, however it may lead to prolonged latency in infected host, with occasional reactivation due to various immunosuppressed states or conditions e.g., Pregnancy, Diabetes, Human Immunodeficiency Virus Infection/Acquired Immunodeficiency Syndrome (HIV/AIDS), bone marrow and organ transplant recipients, and cancer patients on chemotherapeutic agents and individuals receiving immunosuppressive therapy due to the transplantation procedure. HCMV infection can cause an array of damaging clinical effects in the fetus, neonate, and immunocompromised patients; however, in apparently healthy individuals complications are less frequent [4].
Cytomegalic inclusion disease is one of the most frequent causes of congenital infection in infants. The worldwide prevalence (the measurement of all individuals affected by the disease at a particular time) differs substantially in various study population, newborns who develop congenital HCMV infection as a result of primary maternal infection developed during pregnancy or due to reactivation in a seropositive immunosuppressed mother during pregnancy is ~0.64% of live births [5]. Fetal infection can be caused due to maternal primary infections (35 to 50%) and secondary infections (0.2-2%). Amongst primary infection only 5-15% and about 1% in secondary infections lead to clinically significant disease state [6]. It was estimated that approximately, 10 to 15% of congenitally infected babies are symptomatic at birth, clinical presentation varies widely ranging from Intrauterine Growth Retardation (IUGR) to hepatitis with jaundice and hepatosplenomegaly; thrombocytopenia with petechiae; pneumonitis; and severe central nervous system damage with microcephaly, intracerebral calcifications, chorioretinitis, and sensorineural hearing loss and in these symptomatic infants, a mortality rate of ~30% has been reported [7].
Once infected with CMV, the host carries the virus for life. The seroprevalence of CMV among women of childbearing age in developing countries may be very high up to 90% whereas in developed countries it may be as low as 30% [8]. There is a need to determine the seroprevalence of CMV antibody in perinatal women, as the prevalence of congenital infection varies. Therefore, the aim was to screen large number of women in perinatal period with uncomplicated pregnancy for CMV IgM and IgG titers.
Materials and Methods
The retrospective data was analysed over eight years between 2010 to 2018 (and all the results were compiled and analysed in 2019) taking 23,029 maternal samples from women in the perinatal period for CMV antibody (IgM and IgG) at Regrow Biosciences Private Limited, Biocell-Umbilical Cord Blood Stem Cell Bank (approved and licensed by FDA and CDSCO India, where CMV testing on maternal blood sample is mandatory), Lonavala, Pune, Maharashtra, India. For this study written consent was taken for Infectious disease testing. Perinatal period in this study was restricted to 7 days before and after delivery, as per FACT NET CORD guidelines [9,10]. Maternal blood sample was collected to do infectious disease screening prior to long term cryo-preservation of cord blood sample.
Maternal blood sample (5 mL) was aseptically drawn by venipuncture in plain tubes (BD vaccutainers). Serum was separated from blood sample by centrifugation at room temperature at a speed of 1000 to 1300 rpm for 10 minutes. Serum obtained was used for CMV antibody testing by semi-automated ELISA (Enzyme linked immnosorbant assay) method. Sera were tested for IgM and IgG antibodies against CMV by Capture ELISA (DIA.PRO KIT) as per manufacturer’s instructions using ELISA micro plate washer (Micro plate Washer-PW-40 Bio-Rad). Cut-off for positive and negative controls was set as per test procedure. The controls and calibrators were used to validate the procedure recommended by manufacturer of the kit. The colour intensity of the solution in each well was measured using ELISA Micro plate Reader (PR4100-Bio-Rad). Results were analysed on software (Magellan Tracker). Samples with concentration above cut-off were regarded as positive and under cut-off were regarded as negative for CMV antibody.
Results
A retrospective data of total 23,029 women in perinatal period were analysed for CMV IgG and IgM antibodies in this study to determine the seroprevalence of CMV infection in Indian women of childbearing age of 18-45 years. The results disclose that 92.5% women in this study, were found immune (resistant to a particular infection or toxin owing to the presence of specific antibodies or sensitised white blood cells) to CMV infection showing positive result for CMV IgG antibodies and about 7.5% perinatal women were non- immune to CMV showing absence of CMV IgG antibodies. None of them were having any IgM antibodies which is evidence of active infection [Table/Fig-1].
Seroprevalence of CMV in study population.
| Result | Prevalence of CMV-IgG | Prevalence of CMV-IgM |
|---|
| Positive | 21312 | 00 |
| Negative | 1717 | 23029 |
| Total | 23029 | 23029 |
This study also analysed the yearly trend of CMV-IgG positivity for past eight years of cord blood banking and it was observed that incidence of CMV immune cases ranged from 86.82%-95.71%, highest being in the year 2013 whereas non-immune cases ranged from 4.29-13.18%, highest being in the year 2012 [Table/Fig-2].
Yearly incidence of CMV-IgG in study population.
| Year | Total population screened | Prevalence of CMV-IgG |
|---|
| Immune | % | Non-immune | % |
|---|
| 2010 | 441 | 414 | 93.89% | 27 | 6.12% |
| 2011 | 1738 | 1556 | 89.53% | 182 | 10.47% |
| 2012 | 1980 | 1719 | 86.82% | 261 | 13.18% |
| 2013 | 2888 | 2764 | 95.71% | 124 | 4.29% |
| 2014 | 3966 | 3739 | 94.28% | 227 | 5.72% |
| 2015 | 2696 | 2511 | 93.14% | 192 | 7.12% |
| 2016 | 4308 | 3989 | 92.60% | 319 | 7.40% |
| 2017 | 3368 | 3114 | 92.46% | 254 | 7.54% |
| 2018 | 1637 | 1506 | 92.00% | 131 | 8.00% |
| Total | 23,029 | 21,312 | 92.50% | 1717 | 7.50% |
The socioeconomic status and age distribution was also studied amongst non- immune and immune cases [Table/Fig-3]. Since, this cord blood bank is private; all women enrolling for this service belong to relatively higher socioeconomic status. Study populations in the age group of ≥40 years were showing highest seroprevalence whereas women in the age group of <20 years were showing lowest seroprevalence of CMV immunity.
Age distribution amongst CMV non-immune and immune cases.
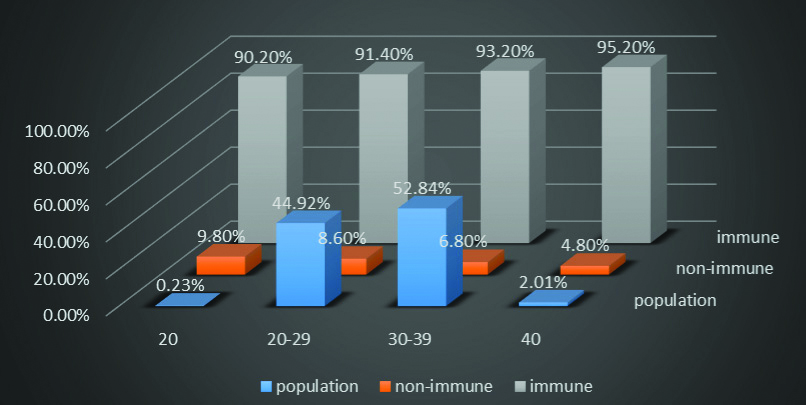
In general, it was observed that incidence of CMV seroprevalence was increasing with increasing age of women from <20 years to >40 years which suggests the likelihood of increased risk of exposure to the infection with increasing age. Most of this study populations were in the age group 30-39 years (52.84%) followed by 20-29 years (44.92%).
Discussion
The most common cause of congenital viral infection in the world is CMV. Both primary and recurrent maternal infection can result in fetal infection, which can result in either intrauterine or postnatal infections. In developed countries, the prevalence of CMV congenital infection of infants varies from 0.3% to 2.3% [9,10], whereas in developing countries it is higher and variable, with some reported prevalence as high as 6-14% [11,12]. The infant may get infection from the mother through intrauterine infection (congenital infection), or through the contact with the infected genital secretions through the birth canal at the time of birth (intra-partum infection), or through breast feeding (postpartum). It was noticed that though there are no associated problems in most of the congenitally infected infants, CMV can still cause the severe neonatal disease and approximately 20% of infected children may suffer from long term disability [13]. In this study, all infants born to women tested for CMV were healthy and had no disability. For this purpose, a program has been followed up for mother and child for a period of six months, in which, any disability in infants was reported by the surveillance team.
During CMV primary infection, humoral response in the host occurs in the form of production of antibodies (initially IgM and later IgG) specifically against multiple CMV proteins like structural tegument proteins (e.g., pp65 and pp150), envelope glycoproteins (predominantly gB and gH and gH/gL multimeric complexes), and Nonstructural proteins such as the IE1 protein. This humoral immunity is crucial in restricting viral dissemination and probably contributes to minimising the clinical manifestations of the disease [14]. IgM antibodies are unable to cross placental barriers and hence they are unable to protect the child from clinical effect of the infection. As most maternal CMV infections are asymptomatic, antenatal women would need to be tested for CMV IgG and IgM antibodies at their first antenatal visit and if found to be sero-negative and susceptible to infection, retesting to be done at a later stage to identify those who seroconvert. For women seropositive at the first visit, the presence of CMV specific IgM might indicate infection early in pregnancy, although pre-conceptual infection could not be excluded as IgM may persist for 3-6 months or even longer after a primary infection. Serological screening would be required to diagnose infections occurring in pregnancy [15]. In this study, sample was collected from women in immediate perinatal period enrolled for cord blood banking and tested for serum CMV IgM and IgG by ELISA method and results suggest that seroprevalence of CMV IgG in this study population (23,029) was 92.5%. This was higher to an earlier study done in western India by Kumar M et al., who reported a seroprevalence of 83.24% [16]. It was also similar to reports from other developing countries but differs from those reported in developed countries [Table/Fig-4] [16-27].
CMV Seroprevalence of different countries in pregnant women [16-27].
| Country | Authors | Population size | Year | Prevalence of CMV-IgG | Prevalence of CMV-IgM |
|---|
| India | Current study | 23,029 | 2019 | 92.57% | 0% |
| India | Kumar M et al., [16] | 370 | 2017 | 83% | 9.46% |
| Nigeria | Akinbami AA et al., [17] | 179 | 2011 | 97.2% | - |
| London | Tookey PA et al., [18] | 20,000 | 1992 | 54.4% | - |
| Nigeria | Hamid KM et al., [19] | 180 | 2014 | 91.1% | - |
| France | Gratacap-Cavallier B et al., [20] | 1018 | 1998 | 51.5% | - |
| Thailand | Fongsarun J et al., [21] | 2101 | 2013 | 86.53% | 0.33% |
| Germany | Lachmann R et al., [22] | 3380 | 2018 | 62% | - |
| Turkey | Sabahattin O et al., [23] | 1652 | 2007 | 94.9% | 0.4% |
| Egypt | El-Nawawy A et al., [24] | 150 | 1996 | 96% | 0% |
| Japan | Nishimura N et al., [25] | 573 | 1999 | 77.5% | - |
| Finland | Mustakangas P et al., [26] | 1088 | 2000 | 70.7% | 4.0% |
| USA | Marshall GS and Stout GG, [27] | 2992 | 2005 | 58% | - |
CMV is a herpes virus, transmitted by intimate contact with secretions of an infected individual. Transmission among individuals is considered to occur primarily through the transmission of saliva or urine; however no quantitative estimations are present for the contribution of different infection routes. As per a study in Netherlands, it was noted that seroprevalence of CMV increase progressively with the age such that, at old age majority of persons in the Netherlands were infected [28]. In this study, similar trend of gradual increase in seroprevalence of CMV was also found in study population with age, highest being in women above 40 years of age.
Cord blood donors (mother) were selected based on many criteria, one of them is that the women (donors) should be free from Transfusion Transmitted Infectious Diseases (TTID). Hence all maternal samples tested for CMV IgM were negative due to stringent screening programme. About 7.5% (1717) of this study population were CMV-IgG negative, which means these woman are potentially susceptible to CMV infection. CMV may lead to prolonged latency in infected host, with occasional reactivation due to various immunosuppressed states or conditions e.g., Pregnancy. Maternal immunity against CMV infection play vital role in protection of fetuses from getting congenitally infected with CMV.
The incidence of CMV infection in humans, is mostly determined by the cultural practices of the population relating to breastfeeding practices, crowding, child rearing arrangements, hygiene standards and practice, the age of onset of sexual activity and the number of sexual partners and the health-care system [29]. Since socioeconomic status determine standard of living and health care, the role of socioeconomic status as a risk factor for acquisition of CMV was confirmed by Anuma ON and Umeoro OU, by linear regression analysis [30].
Socioeconomic conditions in Japan have improved, and the lifestyle in Japan has changed dramatically over the past 20 years. As a result, the prevalence of antibody to CMV has decreased from 87% in 1985 to 75% in 1997 [31]. In the scrutiny of eight years data for incidence rate of CMV-IgG positivity and it was observed that incidence rate of CMV immune cases was almost static. It is observed that, despite the study population belonged to relatively higher socio-economic status, the CMV seroprevalence is matching with other developing countries.
Limitation
The limitation of this study is that, the tests were only performed by ELISA, and confirmation through Chemiluminescent immunoassay (CLIA) was not done.
Conclusion
Since there was no such extensive data available in Indian population, it was aimed to screen large perinatal population which provided more clarity on CMV seroprevalence in Indian perinatal women. Also, the incidence of CMV infection is static over eight years which means personal hygiene and standards of health care system need to be more emphasised and must be practiced routinely.
In this study, it is concluded that, many perinatal women in developing country like India are exposed to CMV infection at very early age in life. Since it can lead to catastrophic and fatal outcome in babies born to mothers with active CMV infection, there is need for routine CMV antibody screening of blood, even in normal pregnant women as well as in immune-compromised individuals and transplant recipients.
Future Recommendations
Further raising awareness of CMV infection among Indian women which may lead to improvement in personal hygiene including pregnant women and elevate standards of healthcare in India which can immediately have impact on the congenital CMV disease acquisition and transmission rate and hence perinatal morbidity.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? NA
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 09, 2019
Manual Googling: Sep 13, 2019
iThenticate Software: Oct 16, 2019 (13%)
[1]. Davison AJ, Dolan A, Akter P, Addison C, Dargan DJ, Alcendor DJ, The human cytomegalovirus genome revisited: Comparison with the chimpanzee cytomegalovirus genomeJ Gen Virol 2003 84:17-28.10.1099/vir.0.18606-012533697 [Google Scholar] [CrossRef] [PubMed]
[2]. Chen DH, Jiang H, Lee M, Liu F, Zhou ZH, Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirusVirology 1999 260:10-16.10.1006/viro.1999.979110405351 [Google Scholar] [CrossRef] [PubMed]
[3]. Sia IG, Patel R, New strategies for prevention and therapy of cytomegalovirus infection and disease in solid-organ transplant recipientsClin Microbiol Rev 2000 13:83-121.10.1128/CMR.13.1.83 [Google Scholar] [CrossRef]
[4]. Crough T, Khanna R, Immunobiology of human cytomegalovirus: From bench to bedsideClin Microbiol Rev 2009 22:76-98.10.1128/CMR.00034-0819136435 [Google Scholar] [CrossRef] [PubMed]
[5]. Acharya D, Immune status in infection by cytomegalovirus in women with bad obstetric historyInt J Infect Microbial 2013 2:3-6.10.3126/ijim.v2i1.7663 [Google Scholar] [CrossRef]
[6]. Kenneson A, Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infectionRev Med Virol 2007 17:253-76.10.1002/rmv.53517579921 [Google Scholar] [CrossRef] [PubMed]
[7]. Michael ME, Aboudy Y, Smetana Z, Tepperberg M, Grossman Z, Laboratory assessment and diagnosis of congenital viral infections: Rubella, cytomegalovirus (CMV), varicella-zoster virus (VZV), herpes simplex virus (HSV), parvovirus B19 and human immunodeficiency virus (HIV)Reprod Toxicol 2006 21(4):350-82.10.1016/j.reprotox.2006.02.00116564672 [Google Scholar] [CrossRef] [PubMed]
[8]. Malm G, Congenital cytomegalovirus infectionsFetal Neonatal Med 2007 12:154-59.10.1016/j.siny.2007.01.01217337260 [Google Scholar] [CrossRef] [PubMed]
[9]. FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration. 6th edition pp:173 [Google Scholar]
[10]. Hyde TB, Schmid DS, Cannon MJ, Cytomegalovirus seroconversion rates and risk factors: implications for congenital CMVRev Med Virol 2010 20(5):311-26.10.1002/rmv.65920645278 [Google Scholar] [CrossRef] [PubMed]
[11]. Bello C, Whittle H, Cytomegalovirus infection in Gambian mothers and their babiesJ Clin Pathol 1991 44(5):366-69.10.1136/jcp.44.5.3661646236 [Google Scholar] [CrossRef] [PubMed]
[12]. Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP, Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain areaChina J Clin Virol 2007 40:180-85.10.1016/j.jcv.2007.08.01817919973 [Google Scholar] [CrossRef] [PubMed]
[13]. Gantt S, Bitnun A, Renaud C, Kakkar F, Vaudry W, Diagnosis and management of infants with congenital cytomegalovirus infectionPaediatr Child Health 2017 22:72-74.10.1093/pch/pxx00229479184 [Google Scholar] [CrossRef] [PubMed]
[14]. La Rosa C, Diamond DJ, The immune response to human CMVFuture Virol 2012 7:279-93.10.2217/fvl.12.823308079 [Google Scholar] [CrossRef] [PubMed]
[15]. Griffiths PD, Stagno S, Pass RF, Smith RJ, Alford CA Jr, Infection with CMV during pregnancy: Specific IgM antibodies as a marker of recent primary infectionJ Infect Dis 1982 145:647-53.10.1093/infdis/145.2.647 [Google Scholar] [CrossRef]
[16]. Kumar M, Nizam MB, Mugunthan M, Seroprevalence of cytomegalovirus infection in antenatal women in a Tertiary Care Center in Western IndiaJ Mar Med Soc 2017 19:51-54.10.4103/jmms.jmms_26_17 [Google Scholar] [CrossRef]
[17]. Akinbami AA, Rabiu KA, Adewunmi AA, Wright KO, Dosunmu AO, Adeyemo TA, Seroprevalence of cytomegalovirus antibodies amongst normal pregnant women in NigeriaInt J Womens Health 2011 3:423-28.10.2147/IJWH.S2485022247628 [Google Scholar] [CrossRef] [PubMed]
[18]. Tookey PA, Ades AE, Peckham CS, Cytomegalovirus prevalence in pregnant women: the influence of parityArch Dis Child 1992 67:779-83.10.1136/adc.67.7_Spec_No.7791325757 [Google Scholar] [CrossRef] [PubMed]
[19]. Hamid KM, Onoja AB, Tofa UA, Garba KN, Seroprevalence of cytomegalovirus among pregnant women attending Murtala Mohammed Specialist Hospital Kano, NigeriaAfr Health Sci 2014 14:125-30.10.4314/ahs.v14i1.1926060468 [Google Scholar] [CrossRef] [PubMed]
[20]. Gratacap-Cavallier B, Bosson JL, Morand P, Dutertre N, Chanzy B, Jouk PS, Cytomegalovirus seroprevalence in French pregnant women: Parity and place of birth as major predictive factorsEur J Epidemiol 1998 14:147-52.10.1023/A:10074507296339556173 [Google Scholar] [CrossRef] [PubMed]
[21]. Fongsarun J, Ekkapongpisit M, Paisan M, Chanthachorn S, Papadopoulos KI, Prevalence of transmissible viral disease in maternal blood samples of autologous umbilical cord blood in a private cord blood bankTransplant Technol 2013 1:110.7243/2053-6623-1-1 [Google Scholar] [CrossRef]
[22]. Lachmann R, Loenenbach A, Waterboer T, Brenner N, Pawlita M, Michel A, Cytomegalovirus (CMV) seroprevalence in the adult population of GermanyPLoS One 2018 13:e020026710.1371/journal.pone.020026730044826 [Google Scholar] [CrossRef] [PubMed]
[23]. Sabahattin O, Sahin Z, Cahit O, Kenan D, Arif G, Seroprevalence of toxoplasma gondii, rubella and cytomegalovirus among pregnant women in Southern TurkeyScand J Infect Dis 2007 39:231-34.10.1080/0036554060097888017366053 [Google Scholar] [CrossRef] [PubMed]
[24]. El-Nawawy A, Soliman AT, el Azzouni O, Amer ES, Karim MA, Demian S, Maternal and neonatal prevalence of toxoplasma and cytomegalovirus (CMV) antibodies and hepatitis-B antigens in an Egyptian rural areaJ Trop Pediatr 1996 42:154-57.10.1093/tropej/42.3.1548699582 [Google Scholar] [CrossRef] [PubMed]
[25]. Nishimura N, Kimura H, Yabuta Y, Tanaka N, Ito Y, Ishikawa K, Prevalence of maternal cytomegalovirus (CMV) antibody and detection of CMV DNA in amniotic fluidMicrobiol Immunol 1999 43:781-84.10.1111/j.1348-0421.1999.tb02470.x [Google Scholar] [CrossRef]
[26]. Mustakangas P, Sarna S, Ammälä P, Muttilainen M, Koskela P, Koskiniemi M, Human cytomegalovirus seroprevalence in three socioeconomically different urban areas during the first trimester: A population-based cohort studyInt J Epidemiol 2000 29:587-91.10.1093/ije/29.3.58710869335 [Google Scholar] [CrossRef] [PubMed]
[27]. Marshall GS, Stout GG, Cytomegalovirus seroprevalence among women of childbearing age during a 10-year periodAm J Perinatol 2005 22:371-76.10.1055/s-2005-87259016215924 [Google Scholar] [CrossRef] [PubMed]
[28]. van Boven M, Van de Kassteele J, Korndewal MJ, van Dorp CH, Kretzschmar M, van der Klis F, Infectious reactivation of cytomegalovirus explaining age- and sex-specific patterns of seroprevalencePLoS Comput Biol 2017 13:e100571910.1371/journal.pcbi.100571928949962 [Google Scholar] [CrossRef] [PubMed]
[29]. Peckham C, Tookey P, Logan S, Giaquinto C, Screening options for prevention of congenital cytomegalovirus infectionJ Med Screening 2001 8:119-24.10.1136/jms.8.3.11911678549 [Google Scholar] [CrossRef] [PubMed]
[30]. Anuma ON, Umeora OU, Seroprevalence of cytomegalovirus antibodies among antenatal clinic attendees in Abakaliki, NigeriaAfr J Med Health Sci 2016 15:24-29.10.4103/2384-5589.183883 [Google Scholar] [CrossRef]
[31]. Hoshiba T, Asamoto A, Yabuki Y, Decreasing seropositivity of cytomegalovirus of pregnant women in JapanJpn J Clin Med 1998 56:193-96. [Google Scholar]