Hospital is a common source of microorganisms. People, both the staff and the patients are at an increased risk of acquiring the infections from the hospital. Nosocomial infection is the clinical infection that develops after 48-72 hours of admission to a hospital resulting from exposure to organisms endemic within the Hospital. These infections were neither overtly present nor within the incubation period in the patients at the time of admission. Infections that are clinically evident after discharge but contracted from the hospital are also a part of this spectrum [1]. There are many ways through which this type of spread is known to occur such as air and fomites, doctors and staff nurse (because of meeting several patients a day), irrational use of antibiotics, improper disposal of waste, ineffective aseptic precautions, improper screening system, improper air condition system [2].
Also, water is a critical component of public health, and failure to supply safe water will place a heavy burden on humanity [3]. Drinking water is the major source of microbial pathogens in developing regions [4]. Furthermore, water may be contaminated by disease causing pathogens from garbage piling, improper waste disposal and excessive use of agriculture chemicals, all of which is very common in Bangalore. Drinking water being transported through distribution networks will be subjected to both chemical and microbiological quality changes [5].
Lack of safe drinking water and proper sanitation leads to number of diseases such as cholera, dysentery, salmonellosis, typhoid and everyday millions of lives are claimed [6]. It is estimated that 1.1 billion people in developing countries have no access to clean water and 2.4 billion people have no sanitation. Consequently, 250 million people are exposed to water borne diseases resulting in 10-20 million deaths every year [7].
Coliform bacteria are a commonly used bacterial indicator of sanitary quality of foods and water. Coliforms include the genera like Citrobacter, Enterobacter, Klebsiella, Escherichia, etc., Presence of these organisms indicate the presence of other pathogenic organisms like viruses, protozoa and parasites.
Fecal coliforms are a group of Fecal Indicator Bacteria (FIB) used to assess water quality throughout the world [8]. FIB is a group of microorganisms of the commensal flora of gut used to indicate the potential occurrence of pathogens in water. This group includes Escherichia coli, Enterococcus, Clostridia, etc.
There are three types of water contamination namely physical contamination, chemical contamination and bacteriological contamination. Contamination of the water supply can occur at the source, the storage tank, the overhead tanks, the pipe lines and ineffective purification systems.
Water is one of the main sources of contamination. There is a dearth of data in South India (Bangalore) regarding the contamination of water supply in a hospital which is beneficial for providing quality of water. Since there have not been many studies conducted to determine the bacteriological analysis of water in Bangalore, this paper aims to determine the prevalence of bacteria along with its bacteriological profile using Hi Media Water Coliform Kit.
The Hi Media Water Coliform Kit is a simple and an easy method to determine the presence of microorganisms. It is a commercially available ready water testing kit in India for speedy and accurate detection of microbes in potable water. The samples that prove to be contaminated will be subjected to culture and sensitivity with antibiotic sensitivity testing to determine the bacteriological profile of the organisms. This method can pave way to determine if the water supply in a hospital can be a significant source of infection contributing to nosocomial and post OP infections.
Materials and Methods
In an attempt to determine the percentage of contamination of water samples along with the determination of bacteriological profile with its prevalence, this descriptive study was undertaken for a period of 1 October 2016 to 30 November 2016 in a tertiary care hospital in Bangalore, Karnataka, India. Institutional Ethics Clearance was taken before commencing the study (Reference number: KIMS IEC/ UG-29/ 2016). Twenty different water source points in the hospital were chosen for the study. Physical and chemical analysis of water was excluded from the study. Isolation of other organisms such as viruses, protozoa, parasites was also excluded from this study. Although E.coli was included in the study, Clostridium and Enterococcus were not included in this study as the study focuses more on gram negative bacteriological profile for water contamination.
Clostridium was excluded as the media and incubator for anaerobic culture was not available at the time of the study. Other organisms such as E.coli, Klebsiella, Enterobacter, Citrobacter, Proteus, NF GNB, Salmonella, Pseudomonas, Acinetobacter, Vibrio were included in the study. Isolation of these organisms can help in the investigation of nosocomial infection. It can help in the treatment of such infections. The data obtained can be significant in case of any nosocomial outbreak ultimately helping in identification of the source of infection.
Materials Used
This study uses single use test kits- Hi Water test kit (K015) supplied by Himedia Laboratories Pvt., Ltd., 20 sets of the kits were used (Batch no. LOT0000280691 and LOT0000264880), each containing 2 sterile bottles and 2 different nutrient Medias ‘A’ and ‘B’. Medium ‘A’ contains peptone (2 gm/Pack); Lactose (0.5 gm/pack); dipotassium hydrogen phosphate (0.15 g/pack); Ferric ammonium citrate (0.075 g/pack); Sodium thiosulphate (0.1 g/pack); Sodium lauryl Sulphate (.01 g/pack); Bromo cresol purple (0.005 g/pack) which helps in detection of Salmonella species, E. coli, Citrobacter species. Medium ‘B’ contains Peptone (1.2 g/pack); sucrose (2 g/pack); Sodium thiosulphate (0.65 g/pack);sodium citrate (1 g/pack); Bile salt (0.6 g/pack); Sodium chloride (1 g/pack); Indicator mix (0.06 g/pack) and helps in detection of Vibrio species. Detection of organism growth is based on change in colour of the medium.
Blood agar plates and Mac Conkey agar plates, Loop for streaking, burner for heating the loop during streaking, Incubator, microscope, spirit, test tubes for biochemical tests with the reagents if any, filter paper impregnated with oxidase reagent.
Points of Water Sample Collection
Neonatal Intensive care unit
Labour room
Surgery Intensive care unit
Dialysis room
Causality
Medicine Intensive care unit
Heart center
Central/Main Intensive care unit
Microbiology lab
UD- Hotel (canteen)
Neurosurgery Operation Theatre Scrub sink
Obstetrics and Gynaecology Operation Theatre Scrub sink
Surgery Major Operation Theatre Scrub sink
Surgery ward
Surgery Minor Operation Theatre Scrub sink
Medicine ward
Orthopaedics minor Operation Theatre Scrub sink
Dermatology minor Operation Theatre Scrub sink
Bronchoscopy room
Orthopaedics ward
Procedure
The 20 different source points of water were chosen for bacteriological water analysis based on the usage and patient inflow after studying the floor plan of the hospital. Each of the chosen 20 points had metal taps that were most commonly used by doctors, staff nurse and patients. Plastic taps were excluded. For collection of water samples, each tap was opened and the water was allowed to flow through for 2 minutes. The tap was then closed. Later, the tap was cleaned with spirit thoroughly immediately after which it was lit using a spirit lamp. This procedure was employed for removing any bacteria that were already present due to their use. After the tap cooled down, it was opened and 100 mL of water was collected after letting the first 30-50 mL of water flow through it. The water was directly collected in the 2 sterile bottles provided in the kit. Once the water was collected, the cap of the bottle was closed immediately to prevent contamination. After collection of water, the nutrient media was added to the two bottles and labeled ‘A’ and ‘B’ respectively and mixed gently in rotatory movements. All the sample bottles were then incubated at 37°C for 24 hours. All the bottles were inspected for colour change after 24 hours. Any bottle with colour change was noted and each of it was cultured on MacConkey agar (MA) and Blood agar (BA) by streaking and all the culture plates were incubated at 37°C for 24 hours.
On day 3, each culture plate was checked for growth [Table/Fig-1]: Different types of colonies were noted and were subjected to the following three biochemical tests.
Indole test
Mannitol Motility test
Triple sugar iron (TSI) test [9]
The test tubes were incubated at 37°C for 24 hours and the test tubes were noted for positive indole test i.e., formation of a cherry red layer after using Kovac’s reagent [Table/Fig-2]. Gas formation/blackish discolouration/colour of ‘But’ and ‘slant’ of the test tube in case of TSI test done using stab and streak method; and motility noted in mannitol motility test.
The organism was narrowed down by understanding the morphology of colonies formed on the agar plates and by three biochemical tests. Further, the sensitivity of the organism to different drugs was determined by Kirby- Bauer method. The organism was streaked using a loop and an antibiotic disc was placed [Table/Fig-3]. This was further incubated at 37°C for 24 hours and the sensitivity was determined by analysing the zone of inhibition [9].
Images showing growth obtained on culture plates.
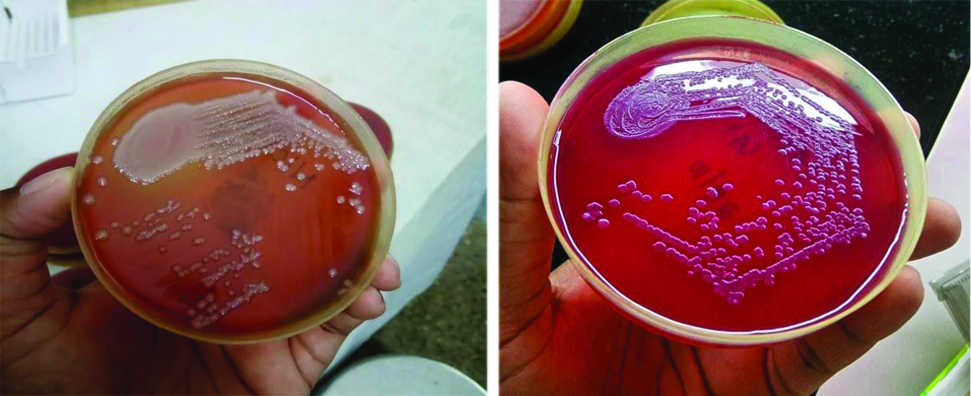
Image showing test tubes for Mannitol motility test, TSI test, Indole test.
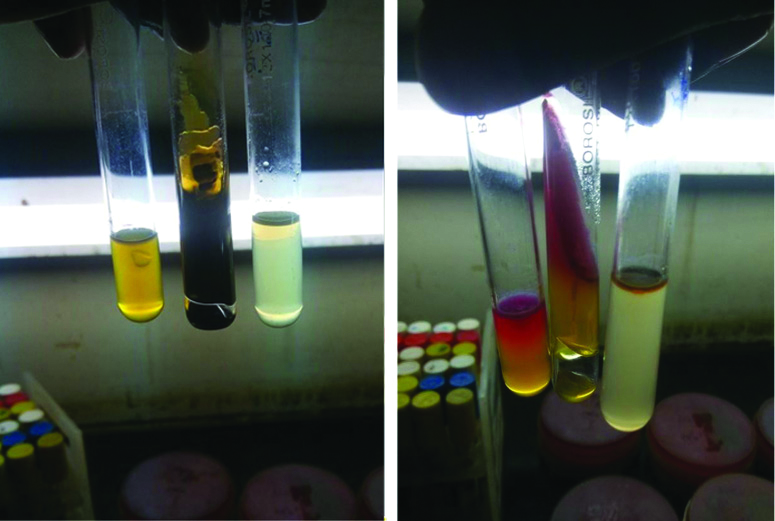
Image showing antibiotic sensitivity testing by disc diffusion method.

Results
The results obtained are tabulated as shown in [Table/Fig-4]. Based on the colonies obtained in the culture plates, biochemical reactions, it was found that the samples were contaminated with Salmonella species, Enterobacter, Klebsiella, E.coli, Citrobacter, Proteus, NF GNB, Pseudomonas, Acinetobacter. Pseudomonas was confirmed by performing Oxidase test which turned out to be positive.
Summary of colonies isolated and noted after subjected to the three different biochemical tests as the samples were taken from 20 different source points of water along with various organisms and its antibiotic sensitivity.
| Sl. No | Source point | Sample | Colour | Colonies isolated | Mannitol | Motility | TSI | Indole | Organism | Antibiotic sensitivity |
|---|
| 1 | NICU | A | Black | LF | P | P | A/A H2S | N | Proteus | Sensitive to all |
| B | Yellow | LF | P | P | K/A | P | E. coli | Sensitive to all |
| PLF | P | N | K/A | N | Klebsiella | Sensitive to all |
| 2 | Labour room | A | Yellow | LF | P | P | K/A | P | E. coli | Sensitive to all except A, AC, CPZ |
| NLF | P | P | K/A | P | E. coli | Sensitive to all except A, AC, CPZ |
| B | Yellow | LF | P | N | K/A | N | Klebsiella | Sensitive to all |
| 3 | Surgery ICU | A | Black | LF | P | N | A/A | P | Klebsiella | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiella | Sensitive to all |
| B | Yellow | NLF | P | P | K/A | P | E. coli | Sensitive to all |
| LF | P | P | K/A | N | Enterobacter | Sensitive to all |
| PLF | N | N | K/A | N | NF GNB | Sensitive to all |
| 4 | Dialysis Room | A | Black | LF | P | N | A/A | N | Klebsiella | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiella | Sensitive to all |
| PLF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| B | Yellow | LF | N | P | A/A H2S | N | Proteus | Sensitive to all |
| NLF | P | P | A/A | P | E. coli | Sensitive to all |
| Swarmimg | N | P | A/A H2S | N | Proteus | Sensitive to all |
| 5 | Casuality | A | Black | LF | P | P | K/A H2S | N | Citrobacter | Sensitive to all except A, AC, IPM |
| B | Black | LF | P | P | K/A | P | E. coli | Sensitive to all except A, AC, IPM |
| 6 | MICU | A | Black | NLF | P | P | A/A | N | Enterobacter | Sensitive to all except CPZ, A, AC |
| LF | P | P | K/A | P | E. coli | Sensitive to all except CPZ, A, AC |
| B | Green | LF | P | P | K/A H2S | N | Salmonellatyphi | Sensitive to all |
| PLF | P | N | K/A | N | Klebsiella | Sensitive to all |
| 7 | Heart center | A | Dark red | LF | P | P | A/A | P | E. coli | Sensitive to all except CPZ, A, AC |
| NLF | P | N | A/A | N | Klebsiella | Sensitive to all |
| B | Yellow | LF | P | P | A/A | N | Enterobacter | Sensitive to all except CPZ, A, AC |
| NLF | P | N | A/A | N | Klebsiella | Sensitive to all |
| 8 | ICU Main | A | Yellow | LF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| NLF | P | P | A/A | N | Enterobacter | Sensitive to all |
| B | Dark Yellow | LF | P | P | K/A | N | Enterobacter | Sensitive to all |
| PLF | P | P | K/A | P | E. coli | Sensitive to all |
| 9 | Microioloy lab | A | Black | LF | P | P | A/A H2S | N | Citrobacter | Sensitive to all except AC and A |
| B | Dark Green | PLF | P | P | K/A | N | Enterobacter | Sensitive to all except AC and A |
| LF | P | P | K/A | P | E. coli | Sensitive to all |
| 10 | UD Hotel | A | Yellow | Spreading LF | N | P | K/K | N | NF GNB | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiella | Sensitive to all |
| B | Black | Oxidase P, NLF | P | P | K/K | N | Pseudomonas | Sensitive to all |
| 11 | Neurosurgery OT | A | Black | LF | P | P | A/A H2S | N | Cirtobacter | Sensitive to all except AC and A |
| PLF | P | P | A/A H2S | N | Cirtobacter | Sensitive to all except AC and A |
| B | Black | LF | P | P | A/A | P | E. coli | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiellapneumonia | Sensitive to all |
| 12 | OBG OT | A | Black | LF | P | P | A/A | N | Enterobacter | Sensitive to all |
| B | Yellow | LF | N | N | K/K | N | Acinetobacter | Sensitive to all |
| 13 | General surgery OT | A | Black | LF | P | P | A/A | P | E. coli | Sensitive to all except CPZ, A, AC |
| NLF | P | P | A/A | P | E. coli | Sensitive to all except CPZ, A, AC |
| B | Black | LF | P | N | A/A | N | Klebsiellapneumonia | Sensitive to all |
| 14 | Surgery ward | A | Green | LF | P | P | A/A | N | Enterobacter | Sensitive to all |
| NLF | P | P | A/A | P | E. coli | Sensitive to all |
| B | Green | | P | N | A/A | N | Klebsiella pneumonia | Sensitive to all |
| 15 | Surgery minor OT | A | Black | LF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| PLF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| B | Dark yellow | LF | P | P | A/A | P | E. coli | Sensitive to all except A, AC, CPZ |
| 16 | Medicine ward | A | Black | LF | P | P | A/A H2S | N | Citrobacter | Sensitive to all except A, AC, IPM |
| NLF | P | P | A/A H2S | N | Citrobacter | Sensitive to all except A, AC, IPM |
| B | Yellow | LF | P | P | A/A | P | E. coli | Sensitive to all except A, AC, CPZ |
| 17 | Orthopaedics minor OT | A | Yellow | LF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| NLF | P | P | A/A H2S | N | Citrobacter | Sensitive to all |
| B | Yellow | LF | P | P | A/A | P | E. coli | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiellapneumonia | Sensitive to all |
| 18 | Dermatology minor OT | A | Yellow | LF | P | P | A/A | P | E. coli | Sensitive to all |
| NLF | P | P | A/A | N | Enterobacter | Sensitive to all |
| B | Yellow | LF | P | P | A/A | N | Enterobacter | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiella pneumonia | Sensitive to all |
| 19 | Bronchoscopy | A | Dark Yellow | LF | P | P | A/A | P | E. coli | Sensitive to all |
| B | Green | NLF | P | P | A/A | N | Enterobacter | Sensitive to all except CXM |
| 20 | Orthopaedics ward | A | Yellow | LF | P | P | A/A | N | Enterobacter | Sensitive to all except A, AC, CPZ |
| NLF | P | P | A/A | P | E. coli | Sensitive to all except A, AC, CPZ |
| B | Yellow | LF | P | N | A/A | N | Klebsiella pneumonia | Sensitive to all |
| NLF | P | N | A/A | N | Klebsiellapneumonia | Sensitive to all |
1. Percentage of Contamination
Since colour change has been observed in all samples, both ‘A’ and ‘B’ and culture has revealed that there is growth in all samples, there is 100% contamination of water at all source points of the hospital [Table/Fig-5].
Image showing contamination in all the bottles.
 |
|---|
| No. of samples | Growth positive | Growth negative | Percentage |
|---|
| 20 | 20 | 0 | 100% |
2. To Identify the Prevalence of Bacteria (Coliform and Others) in the Water Sample
Eighteen samples were contaminated with E.coli (including neurosurgery OT) indicating a prevalence of 90%, 13 with Klebsiella, 9 with Enterobacter, 8 with Citobacter, 2 with Proteus, 2 with NF GNB,1 with Salmonella (seen in MICU), 1 with Pseudomonas (seen in UD Hotel), 1 with Acinetobacter. Isolation of organisms other than coliforms indicates that there is a good chance of significant spread of nosocomial infection through water. There is an increased risk of nosocomial infection, especially in immuno compromised patients creating difficulty in treating patients and increased mortality and morbidity [Table/Fig-6].
Indicates the prevalence of the organisms.
| Organism | Samples positive | Total number of samples | Prevalence |
|---|
| E. coli | 18 | 20 | 90% |
| Klebsiella | 13 | 20 | 65% |
| Enterobacter | 9 | 20 | 45% |
| Citrobacter | 8 | 20 | 40% |
| Proteus | 2 | 20 | 10% |
| NF GNB | 2 | 20 | 10% |
| Salmonella | 1 | 20 | 5% |
| Pseudomonas | 1 | 20 | 5% |
| Acinetobacter | 1 | 20 | 5% |
| Vibrio | 0 | 20 | 0% |
3. To Determine the Bacteriological Profile of the Organisms Present
Most of the isolates were sensitive to all drugs. However, a few isolates of E.coli, Citrobacter, Enterobacter were resistant to Ampicillin, Amoxicillin- clavunalic acid, Imipenem, Cefoperazone, Cefuroxime. Antibiotic testing is essential as it helps in further management of the patients and also helps to analyse the severity of contamination. Such sensitivity patters shows us that there is an indiscriminate use of antibiotics and genetically multi drug strains are emerging.
Discussion
Hospital water quality is directly related to patient’s health. A similar study on microbiological status of drinking water suggested that there was contamination with S. aureus, S. intermedius, S. felis and S. saccharolyticus which was sensitive to erythromycin, tetracycline, norfloxacin, ciprofloxacin. The antibiotic sensitivity was done by disc diffusion method [9]. In another similar study done in Yaounde University teaching hospital which concluded that 75% of water samples of hospital were culture positive with Burkholderia cepacia, Klebsiella, Acinetobacter, Citrobacter, Serratia causing increased risk of nosocomial infection [10].
The above results obtained in this study indicate that E.coli was a major contaminant of water in the hospital followed by Klebsiella, Enterobacter, Citrobacter, Proteus, NFGNB, Salmonella, Pseudomonas, Acinetobacter. Isolation of Salmonella poses increased risk of Typhoid fever. Pseudomonas is notorious to cause infection and sepsis, more commonly in immunocompromised patients. With a contamination rate of 100%, one can infer that the existing water treatment of the hospital is not up to the mark showing that the water is unfit for use and could be one of the potential sources of infections in patients. This could be because of patients coming in direct contact with water, doctors washing their hands and touching patients, scrubbing before surgery. Post-operative patients, Diabetics and immune-compromised patients are at a higher risk of acquiring these diseases, possibly increasing the disability and morbidity of the patient with increase in duration of hospital stay and delayed recovery time, creating more trouble to patients who probably cannot afford the extended treatment. The above results were discussed in detail with the hospital management. The concerned authorities promised to take necessary action to reduce water contamination by cleaning the water tanks and installation of new water purification systems. Studies suggest that there is an increased risk of postoperative infections, mainly due to water. Prevention is always better than cure. With that in mind, measures have to be taken to prevent possible risk of transmission of communicable diseases and help prevent further complications, reduce mortality and morbidity of patients coming to the hospital. The following steps can be taken at different levels.
Doctors can use sterile gloves while examining the patients. In the Operation theatre, after scrubbing, spirit can be used. Spirit should also be used before and after examining a patient to prevent spread of infection. Furthermore, each department of the hospital has to take initiative to clean the water taps regularly, fumigate the wards and operation theatre regularly. Regular water testing helps in monitoring of the water quality. With the advancement in science and technology, use of biosensors and spectrophotometric methods are now trending for detection of microorganisms. Proper waste disposal and Proper sewage treatment plan has to be maintained by using closed dustbins, frequent cleaning of water tanks, changing of old pipes and keeping the tanks closed. Good hygiene is also very essential. Studying the patterns of infection in patients can help identify the root cause and source of infection. The hospital administrative department can install automatic taps that reduces the infection as it eliminates manual opening of taps.
Regular inspection by the hospital authorities is needed. The hospital can install and use the techniques for water treatment such as Boiling, UV light, chemicals like chlorine, electronic purification, and filtration by reverse osmosis.
According to WHO, about 1.1 billion people globally drink unsafe water and the vast majority of diarrhea associated diseases reported across the globe is attributable to unsafe water, sanitation and hygiene. Since water-borne diseases are still major health burden in many parts of the world, allowing the water to come to a rolling boil followed by cooling is sufficient to inactivate bacteria, virus and protozoa [11].
This method is simple to practice, easy and cost effective. UV Light is an expensive but good bactericidal agent. Chlorine, a commonly used chemical method of water purification is good bactericidal agent. In chlorination of water, chlorine forms hydrochloric acid and hypochlorous acid which is bactericidal in nature. It is available in solid, liquid and gaseous forms. Hydrogen peroxide may be used in emergencies. Oxidation using ozone is also done but it is toxic and expensive. Filtration of water, however, is one of the best methods for water filtration in large scale [12].
Although mechanical filters such as ceramic filters, Katadyn filters, berkefeld filter, chamberland filter can used, reverse osmosis is one of the most efficient methods of water purification and cost efficient on a large scale. It uses a membrane with microscopic holes that filter out the bacteria. The mechanism of action can be broken down into 5 stages. In stage one, the sand, silt and dust particles are removed. In the second stage, there is an activated carbon filter that removes all the chemical impurities. In stage three, there is a gag filter that removes the bad taste and odour producing substances. In stage four, the water passes through 0.0001 mm pore allowing only water molecules to pass through. In stage five, Bacteriostatic silver impregnated activated carbon prevents the growth of bacteria and removes the odour [12]. One or more of the above methods can be used to keep the water clean and fit for use.
Water is a main source of hospital acquired infections. Maintaining water fit for use will reduce the number for hospital acquired infections. Installation of reverse osmosis in the hospital premises is the best method for water purification [13]. It paves way for preventing the occurrence of multi drug resistance bacteria. It has good efficiency and is a one time investment. The results are worthy.
Limitation
This study does not include the physical and chemical contamination of water. Furthermore, Viruses, Parasites and protozoa were excluded from study due to lack of facility.
Conclusion
One or more of the above methods, individually or in combination could be used to treat water. Water from different points of the hospital has to be taken again and culture and sensitivity must be done to check the efficacy of the system.
Proper water facilities are an essential requisite to ensure infections do not spread through contaminated water. The Hospital should inspect the water sources using the floor map and look for points of possible contamination and fix it. This study also provides a cautionary tool to maintain a proper sanitation and hygiene in a health care center so as to prevent occurrence of diseases in already immunosuppressed patients. The other methods like boiling, chlorination etc., can also be employed. Water quality in a hospital should be maintained strictly to help build a better quality of life of patients.
List of Abbreviations:
E- coli- Escherichia Coli
SICU- Surgery Intensive Care unit
MICU- Medicine Intensive Care unit
ICU- Intensive care unit
OBG- Obstetrics and Gynaecology
OT- Operation Theatre
BA-Blood agar
MA- MacConkey agar
NICU- Neonatal Intensive care unit
MM- Mannitol Motility
TSI- Triple Sugar Iron
LF- Lactose Fermenting
NLF- Non Lactose fermenting
NF GNB- Non fermenting gram negative bacilli
P- Positive
N- Negative
A/A- Acid/Acid
K/K- Base/Base
A/K- Acid/Base
K/A- Base/Acid
H2S- Hydrogen Sulphide
CPZ- Cefoperazone
A- Ampicillin
AC- Amoxicillin- clavulanic acid
IMP- Imipenem
CXM- Cefuroxime
Post- OP- Post Operation
PLF- Pale Lactose Fermenting