Recurrent Abortions Presenting with Congenital Absence of Inferior Vena Cava in a Young Female
Surabhi Gupta1, Neha Nischal2
1 Resident, Department of Radiology, GB Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
2 Senior Resident, Department of Radiology, GB Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Surabhi Gupta, C-152 (Second Floor), Sarvodaya Enclave, New Delhi-110017, India.
E-mail: surabhig27@gmail.com
Inferior Vena Cava (IVC) agenesis is an unusual cause of deep vein thrombosis, particularly in young individuals. We report a case of a 28-year-old Indian female presenting with recurrent abortions, multiple episodes of deep vein thrombosis and clinically positive for lupus anticoagulant. CECT abdomen demonstrates complete/partial agenesis of IVC and delineates the superficial and deep collateral pathways which develop subsequent to IVC agenesis.
Collaterals, Lupus anticoagulant, Thrombosis
Case Report
A 28-year-old female (G4P0L0), post abortal status presented to the gynecological emergency with complaints of bleeding per vaginum since last 10 days associated with passage of clots and pain in lower abdomen. The patient had a history of recurrent abortions. The patient also complained of multiple episodes of swelling in bilateral legs. She was a non-smoker, and there was no significant family history. She had no upper or lower gastrointestinal symptoms. There was no weight loss. There was no history of cardio-respiratory disease. The patient was positive for treponemal serology, with reactive VDRL test and a value of 67.50 for lupus anticoagulant. There was associated deficiency of protein S with normal values of protein C and no Factor V leiden mutation.
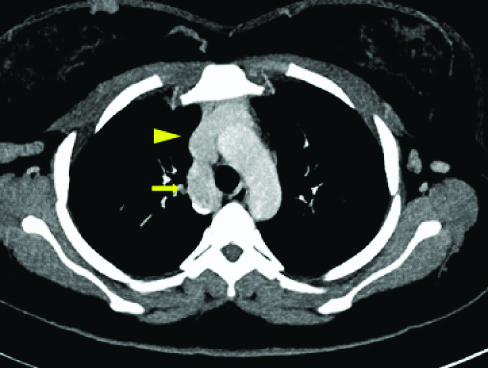
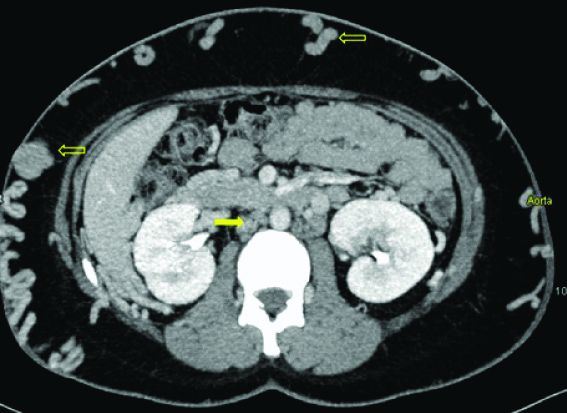
On examination, there were bilateral lower limb varicose veins and multiple tortuous, hard, palpable veins were noted over the entire abdomen. An initial Ultrasound (US) pelvis was performed which revealed blood clots in the endometrial cavity. US Doppler for lower limbs revealed thrombus in bilateral superficial femoral veins with numerous varicose veins. The CT scan revealed dilated tortuous inferior epigastric veins [Table/Fig-1,2 and 3] present bilaterally along with dilated ascending lumbar veins [Table/Fig-4] and dilated azygous vein draining into superior vena cava [Table/Fig-5]. The CT scan also revealed non-visualisation of the entire inferior vena cava with multiple collaterals in the antero-lateral abdominal wall [Table/Fig-4,6] and retroperitoneum. A final diagnosis of congenital absence of IVC with recurrent Deep Vein Thrombosis (DVT) was made. The patient was given low molecular weight heparin initially, till an International Normalised Ratio (INR) between 2-3 was achieved and subsequently discharged on oral anticoagulant therapy. No surgical management was undertaken.
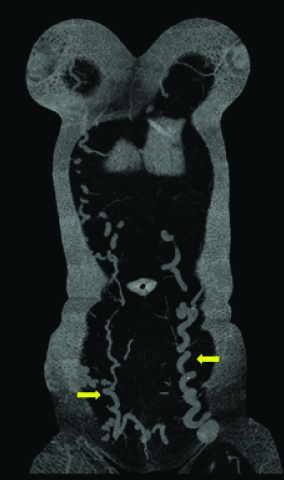
Coronal MIP images of the abdomen and pelvis showing the dilated and tortous inferior epigastric veins (arrows) bilaterally.
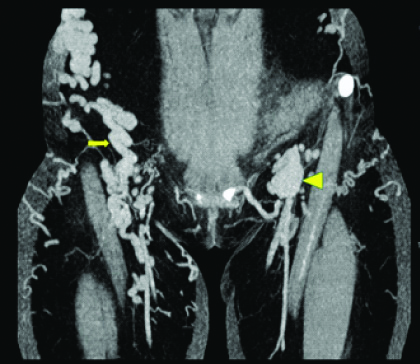
Coronal MIP image of pelvis and thigh show presence of dilated and tortous inferior epigastric vein on right side (arrow) and dilated external iliac vein on left side (arrow head).
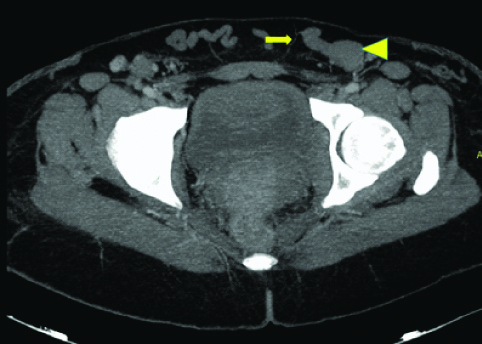
Axial MIP image at the level of hip joint shows external iliac vein (arrow head) draining into inferior epigastric vein (arrow), both which are dilated.
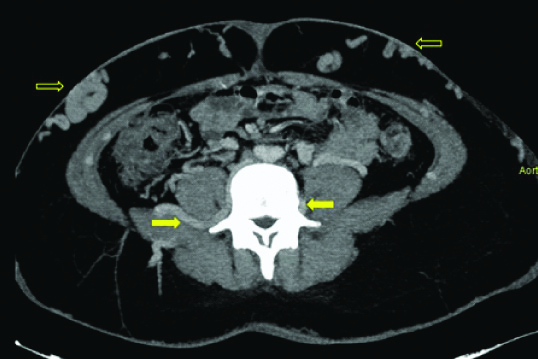
Axial MIP image of abdomen reveals dilated ascending lumbar veins (arrows) present bilaterally. Also, note multiple dilated anterior abdominal veins (open arrows).
Axial MIP image at the level of aortic arch shows the dilated azygous vein (arrow) draining into superior vena cava (arrow head).
Axial MIP image at level of renal hilum shows the absence of IVC (arrow). Note made of multiple superficial abdominal wall collaterals (open arrows).
Discussion
The prevalence of IVC anomalies is 0.07%-8.7% in the general population and complete agenesis of IVC is a rare but well recognised entity [1,2]. The development of IVC begins around 6th-8th week of embryogenesis. The IVC is formed from a complex mechanism involving the appearance, anastomosis and regression of three paired primitive veins namely the posterior cardinal, the subcardinal and the supracardinal veins [3]. Any disturbance in this embryologic process can lead to IVC anomalies, which include, double IVC, left IVC, retrocaval ureter, interruption of IVC with azygous/hemiazygous continuation, porto-caval shunts, membranous obstruction of IVC, and hypoplastic segments of IVC. Agenesis of IVC is the rarest congenital malformation of IVC [4].
Agenesis of the IVC can occur in three different situations:
Absence of the suprarenal IVC results from failure to form the right subcardinal vein. The hepatic segment drains directly into the right atrium, and the blood from the infrarenal IVC returns to the heart through the azygos and hemiazygos veins. Cardiac and visceral anomalies may be associated [5].
Absence of the infrarenal IVC with preservation of the suprarenal segment implies a failure in the development of the right supracardinal vein [6].
Absence of the entire IVC, as in our patient’s case, suggests that all three paired vein systems failed to develop properly [7].
Although most authors believe that these are embryologic events, a number of recent studies, however, suggest that the absence of the IVC, especially of a segment, may be due to perinatal IVC thrombosis, which leads to its subsequent hypoplasia and agenesis [8]. The congenital risk factors for thrombosis include deficiencies of protein C, protein S, antithrombin III and gene mutations of prothrombin and factor V Leiden and presence of lupus anticoagulant. Our case showed reactive VDRL with high values for lupus anti-coagulant.
Generally, individuals with IVC agenesis develop extensive collaterals in order to compensate for the anomaly. The venous return through these collaterals is inadequate resulting in chronic venous hypertension in the lower extremities, causing venous stasis, which subsequently precipitates venous thrombosis.
Inferior Vena Cava Agenesis (IVCA) as a cause of DVT has a prevalence of 0.0005% to 1% in general population [9], much lower than in individuals under 30 years, where IVC agenesis accounts for approximately 5% cases of DVT [10].
The collateral pathways that develop in individuals with IVC anomalies are [11]:
Deep pathway (most common): It involves the ascending lumbar veins mainly. These anastomose with azygous and hemiazygous veins which drain into the superior vena cava and finally the right atrium.
Superficial pathway (common): Blood drains from external iliac veins to the inferior epigastric veins. The flow continues to the superior epigastric veins and then through the internal mammary to the brachiocephalic veins, finally draining into superior vena cava and right atrium subsequently.
Portal pathway (less common): Venous blood from the internal iliac veins returns to the haemorrhoidal plexus and through the superior rectal vein it reaches the inferior mesenteric, then the lienal vein and porta.
Intermediate pathway (rare): It develops in cases with absence of the infrarenal part of the IVC where the renal and suprarenal portions are preserved. In such cases the blood could return through the internal iliac veins to the uterine/ prostatic plexuses and then through the ovarian/pampiniform plexus to the left gonadal vein which flows into the left renal vein.
The deep and superficial pathways of collateral venous drainage were seen in our case.
Since DVT is a common presentation in such cases, often ultrasound is the first imaging modality used. However, IVC being a retroperitoneal structure is difficult to trace along its entire course.
The imaging method of choice is Contrast-Enhanced Computed Tomography (CECT) [11] in which venous phase is essential. CECT chest and abdomen demonstrates the region of entire length of IVC drainage, and hence clearly demonstrates complete agenesis or segmental IVC agenesis. It is useful in defining the segment of agenesis or the level of obstruction in cases of thrombosis. All of the major collaterals can be assessed in a well-performed examination. Multiplanar reformations and MIP projections are very useful and aid to the diagnosis. CECT chest and abdomen can also show presence of enhancing tumour in IVC, any extrinsic compression on IVC and other organ system which can be a part of syndrome associated with IVC agenesis. Differential diagnosis includes chronic obstruction of the IVC. It might be due to thrombosis or extrinsic compression of primary tumours like Sarcoma or Leiomyomatosis.
There have been very few case reports highlighting absence of IVC as a cause of recurrent DVT, like case by Paddock M et al., who reported partial IVC agenesis as opposed to complete agenesis as in our case and one more case by Bolocan A et al., in which the patient had a significant delay in diagnosis of IVC agenesis as a cause of recurrent DVT, also seen in our case, and had undergone surgery (B/L stripping of saphenous vein) for varicose veins, which has little role in such cases [12,13]; and one by Iqbal J et al., which showed association of atrophic left kidney with IVC agenesis, not seen in our case [8]. Our case considers the rare anomaly of complete IVC agenesis as a cause of recurrent DVT and abortions in the Indian subcontinent which to the best of my knowledge has not been done in the past.
There are no specific treatment options available as IVC agenesis associated DVT is a rare condition. Aim of the therapy is to prevent any further progression and recurrence of thrombosis. Patients with DVT as a result of agenesis of IVC should start with anticoagulation therapy as early as possible. Surgical intervention has restricted role in treating these IVC anomalies. Concurrent pulmonary embolism is not a frequent finding in DVT due to absence of IVC and therefore prophylactic IVC filters are not recommended [12].
Conclusion
It is reasonable to consider IVC agenesis as a differential diagnosis in young patients (less than 30 years of age) presenting with extensive DVT in the absence of congenital/acquired venous thromboembolism risk factors. CECT scan or MRI should be considered in diagnosing IVC anomalies and for delineating the major collateral pathways of venous return.
Author Declaration:
Financial or Other Competing Interests: No
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Jul 08, 2019
Manual Googling: Sep 02, 2019
iThenticate Software: Oct 07, 2019 (15%)
[1]. Obernosterer A, Aschauer M, Schnedl W, Lipp RW, Anomalies of inferior vena cava in patients with venous thrombosisAnn Intern Med 2002 136(1):37-41.10.7326/0003-4819-136-1-200201010-0000911777362 [Google Scholar] [CrossRef] [PubMed]
[2]. Ackula H, Mosalpuria K, Tandra P, Congenital agenesis of inferior vena cava as a rare cause of deep vein thrombosis: Case report and review of the literatureCase Reports in Internal Medicine 2016 3(3):74-77.10.5430/crim.v3n3p74 [Google Scholar] [CrossRef]
[3]. Bass JE, Redwine MD, Kramer LA, Huynh PT, Harris JH Jr, Spectrum of congenital anomalies of the inferior vena cava: Cross-sectional imaging findingsRadiographics 2000 20(3):639-52.10.1148/radiographics.20.3.g00ma0963910835118 [Google Scholar] [CrossRef] [PubMed]
[4]. Kandpal H, Sharma R, Gamangatti S, Srivastava DN, Vashisht S, Imaging of the Inferior vena cava: A road less traveledRadioGraphics 2008 28(3):669-89.10.1148/rg.28307510118480478 [Google Scholar] [CrossRef] [PubMed]
[5]. Sneed D, Hamdallah I, Sardi A, Absence of the retro hepatic inferior vena cava: What the surgeon should knowAm Surg 2005 71(6):502-04. [Google Scholar]
[6]. Gayer G, Zissin R, Strauss S, Hertz M, IVC anomalies and rightrenal aplasia detected on CT: A possible link?Abdom Imaging 2003 28(3):395-99.10.1007/s00261-002-0090-712719912 [Google Scholar] [CrossRef] [PubMed]
[7]. Singh K, Poliquin J, Syversten G, Kohler DO, A rare cause of venous thrombosis: Congenital absence (agenesis) of the inferior vena cavaInt J Angiol 2010 19(3):e110-12.10.1055/s-0031-127837722477618 [Google Scholar] [CrossRef] [PubMed]
[8]. Iqbal J, Nagaraju E, Congenital absence of inferior vena cava and thrombosis: A case reportJournal of Medical Case Reports 2008 2:4610.1186/1752-1947-2-4618269760 [Google Scholar] [CrossRef] [PubMed]
[9]. Lambert M, Marboeuf P, Midulla M, Trillot N, Beregi JP, Mounier-Vehier C, Inferior vena cava agenesis and deep vein thrombosis: 10 patients and review of the literatureVascular Medicine 2010 15(6):451-59.10.1177/1358863X1039135521183652 [Google Scholar] [CrossRef] [PubMed]
[10]. Ruggeri M, Tosetto A, Castaman G, Rodeghiero F, Congenital absence of the inferior vena cava: A rare risk factor for idiopathic deep-vein thrombosisThe Lancet 2001 357(9254):44110.1016/S0140-6736(00)04010-1 [Google Scholar] [CrossRef]
[11]. Kalchev E, Popova RD, Valchev G, Balev B, Varna BG, Congenital absence of inferior vena cavaECR 2015/C-1980DOI: 10.1594/ecr2015/C-1980 [Google Scholar]
[12]. Paddock M, Robson N, The curious case of the disappearing IVC: A case report and review of the aetiology of inferior vena cava agenesisRadiology Case 2014 8(4):38-47.10.3941/jrcr.v8i4.157224967034 [Google Scholar] [CrossRef] [PubMed]
[13]. Bolocan A, Ion D, Ciocan DN, Pãduraru DN, Congenital agenesis of the inferior vena cava-cause of deep vein thrombosisChirurgia 2014 109:832-36. [Google Scholar]