Introduction
In a developing country like India, patients of pulmonary Tuberculosis (TB) form an important subset of individuals requiring smoking cessation counselling and pharmacotherapy. Smoking increases risk of Mycobacterium Tuberculosis (MTB) infection, the risk of progression from infection to disease and the risk of death from TB. Smoking cessation counselling and pharmacological therapy help in improving outcomes of TB treatment, reduce transmission of TB and improve the quality of life of TB patients.
Aim
To assess the effectiveness of smoking cessation counselling combined with bupropion in patients of pulmonary tuberculosis using urinary cotinine levels. The study was a prospective interventional study, carried out at Out Patient Department (OPD) and inpatient setting of a tertiary care respiratory centre in an urban setting between the period of July 2016 to June 2019.
Materials and Methods
Seventy-six pulmonary TB patients who were active tobacco smokers and fulfilled the inclusion criteria were recruited for the study. Subjects received smoking cessation counselling for one year along with tab bupropion for first three months. Effectiveness of the interventions was assessed by regular follow-up visits at one month, three months, six months and twelve months post-initiation of therapy. Data analysis was done by using SPSS version 17.0 and Microsoft Excel. Chi-square test was used and p-value <0.05 was considered significant.
Results
A total of 63 subjects were eligible for statistical analysis. Abstinence rates with bupropion and smoking cessation counselling at 12 months of follow-up were found to be 15.9%. Abstinence rates were found to be higher in patients with low nicotine dependence.
Conclusion
The study will provide data on smoking cessation with counselling and bupropion in Indian patients suffering from pulmonary TB.
Fagerstrom, Mycobacterium tuberculosis, Nicotine dependence
Introduction
Tobacco smoking is a great burden on mankind in terms of preventable morbidity and mortality. The World Health Organisation (WHO) has estimated that there are 1.1 billion smokers worldwide, out of which 80% reside in low- and middle-income countries. Tobacco kills about half of its users- nearly 8 million people every year [1]. The Global Adult Tobacco Survey (GATS 2) in 2016-17 showed that 42.4% of men, 14.2% of women constituting 28.6% of the adult population use tobacco in some form in India [2]. Smoking is a risk factor for six of the eight leading causes of deaths in the world, contributing significantly to the burden of three main tobacco related illnesses-Chronic Obstructive Pulmonary Disease (COPD), Coronary Artery Disease (CAD) and cancer of different parts of the body including lungs. Lung cancer rates in smokers climb to 5-10 times of those in non-smokers and tobacco smoking causes more than 80% of all lung cancers in developed countries [3]. Though up to 55% of smokers were planning to quit as per GATS 2 study, only a few eventually succeed. Smoking is a notoriously difficult addiction to quit, due to highly addictive nature of nicotine. Nicotine causes physical dependence and tolerance [4].
In developing countries, where awareness about hazards of smoking is low, initial steps towards a tobacco free society include smoking cessation counselling and pharmacotherapy. Smoking cessation counselling in the form of 5 A’s (ask, advise, assess, assist and arrange) forms the bedrock of any smoking cessation program. Brief advice (for 3-5 minutes) from a doctor given to all smokers to encourage them to make an attempt to quit is effective in promoting smoking cessation. This advice leads to smoking cessation in 1-3% smokers for six months [5].
Since the 1980’s, various pharmacological interventions including Nicotine Replacement Therapy (NRT), bupropion and varenicline have become available. NRT is available in various forms including gums, lozenges, patches, inhalers and nasal sprays. It substitutes one form of nicotine delivery with another. The aim of NRT is to wean off smokers by reduction in frequency and dose of nicotine over time and also by mitigating nicotine withdrawal symptoms. Abstinence rates at the end of one year of using NRT are approximately 10% [6].
Affect or mood exerts strong effects on the individual’s choice to use nicotine [7]. Affective disorders are more common in smokers as compared to non-smokers and individuals with a negative affect are more likely to be smokers and are less likely to quit smoking [8]. These effects may be related to changes in dopaminergic activity in the brain. Therefore, antidepressants or anxiolytics may be efficacious in smoking cessation. Bupropion is an atypical aminoketone antidepressant which acts by inhibiting uptake of dopamine and noradrenaline in the mesolimbic dopamine system. Hurt and colleagues conclusively showed that bupropion is an effective pharmacological agent for smoking cessation using a Randomised Controlled Trial (RCT). Continuous abstinence rates at the end of 12 months were 23% in subjects who received Tab. Bupropion 300 mg/day for 7 weeks and 12% in subjects who received placebo [6]. Another pharmacological agent Varenicline is a partial agonist at α4β2 acetylcholine receptors and acts by reducing smokers’ urge to smoke and their withdrawal symptoms. The results of a meta-analysis of published studies done by Fiore and colleagues indicate that providing counselling in addition to medication significantly enhanced treatment outcome compared to medication alone. The Odds Ratio (OR) (95% CI) for medication alone was 1.0 and estimated abstinence rate (95% CI) was 21.7%, whereas for medication and counselling the estimated OR (95% CI) was 1.4 and estimated abstinence rate (95% CI) was 27.6% [9].
Due to its cost effectiveness, Bupropion is the pharmacological agent of choice in resource limited settings. Urinary cotinine levels and Carbon monoxide levels are commonly used to validate self-reported abstinence. Cotinine is an alkaloid found in tobacco and is also the predominant metabolite of nicotine. Cotinine is used as a biomarker for exposure to tobacco smoke. In a study done on urinary cotinine levels in India, Cotinine levels were significantly high with all forms of tobacco use viz., cigarette, beedi, hookah and chewing tobacco, in comparison to those in non-smokers and passive smokers [10]. Cotinine has an average half-life of about 16 hours. Active smokers excrete cotinine in the range of 1000-8000 ng/mL in urine. With two weeks of abstinence, urinary cotinine values fall below 50 ng/mL. Rapid urinary cotinine test kits used for the study had a cut off value of 200 ng/mL [11].
In a developing country like India, patients of Pulmonary Tuberculosis (TB) form an important subset of individuals requiring smoking cessation counselling and pharmacotherapy. Tuberculosis places a heavy burden on economies the world over, especially in developing countries. There were an estimated 10 million new cases of TB worldwide and 1.6 million people died from the disease [12]. India shares a disproportionately high chunk of this burden with annual incidence of TB of 2.7 million in 2017 and 0.42 million deaths [13]. Smoking increases risk of Mycobacterium Tuberculosis (MTB) infection, the risk of progression from infection to disease and the risk of death from TB [14]. Thus, diagnosis of TB is an important point for imparting behavioural counseling in order to change patient’s smoking habits, with the patient more likely to accept the behaviour change at this juncture. Smoking cessation counselling and pharmacological therapy help to improve outcomes of TB treatment, reduce transmission of TB and improve quality of life of TB patients [15]. The present study is being undertaken to assess the effectiveness of smoking cessation counselling combined with bupropion in patients of pulmonary TB. This study will provide data on effectiveness of smoking cessation counselling and bupropion and help in formulating smoking cessation strategies for TB patients.
Materials and Methods
This prospective interventional study was carried out at Out Patient Department (OPD) and inpatient setting of a tertiary care respiratory centre in the urban setting between the period of July 2016 to June 2019.
Inclusion Criteria
Newly diagnosed patients of drug-susceptible pulmonary TB started on ATT, subjects actively smoking tobacco during last one month (Cut off for defining current smokers was taken as having smoked even once in last one month), Subjects motivated to quit smoking, Subjects who gave consent for smoking cessation therapy and to be a part of the study, Subjects with age 18 years or more.
Exclusion Criteria
Subjects not motivated to quit smoking; Subjects having:(a) Seizure disorder, (b) Unstable cardiac or renal condition, (c) Stroke or brain tumour; Conditions like Pregnancy or lactation and any psychiatric illness; Current substance abuse/alcohol dependence and Subjects consuming tobacco in any other form besides smoking.
Methodology
Seventy six pulmonary TB patients who were active tobacco smokers during last one month and fulfilled the inclusion criteria were recruited for the study. Ethical committee approval was taken before study initiation (Ethical Committee Clearance certificate number 07/2019). Written informed consent was obtained from all subjects preceded by an explanation about nature, purpose of the study and adverse effects of the interventions. Baseline characteristics of all subjects were noted in a proforma in the form of subjects’ demographic particulars, nicotine dependence, microbiological and biochemical parameters. Nicotine dependence was measured using a simplified Fagerstrom Test for Nicotine Dependence (FTND) for ease of statistical analysis. Nicotine dependence was classified into 3 levels: low (0 to 4 points), moderate (5 points) and high (6 to 10 points) [16]. FTND does not take into consideration the type of smoking viz cigarette, beedi or pipe. Subjects received regular smoking cessation counselling for one year along with tab bupropion for first three months. Therapy was started seven days prior to the patient’s quit date with 150 mg bupropion once daily in the morning for 3 days. If this was tolerated, 150 mg was added in late afternoon. Patients were advised to reduce smoking but were allowed to smoke for first week of therapy and stop smoking from the quit date. Effectiveness of the interventions was assessed by regular follow-up visits at one month, three months, six months and twelve months post initiation of therapy. Frequency of adverse effects of bupropion was also studied. All subjects were continued on smoking cessation counselling and tab bupropion for first three months irrespective of smoking status. On follow-up at three months when treatment phase with bupropion was completed, subjects were assessed for abstinence on the basis of self-reported abstinence and rapid urinary cotinine test results. Those subjects who were found to be non-abstinent at three months of follow-up were labelled as non-abstinent and not followed up further. Subjects who were found to be abstinent at the end of three months of follow-up were followed up at six months and twelve months and further assessed for continued abstinence and imparted smoking cessation counselling during follow-up visits. Any subject found to be not abstinent during follow-up at six months and twelve months were labelled non abstinent and not followed up any further. Subjects who reported being abstinent during follow-up at twelve months were labelled abstinent.
Statistical Analysis
Data analysis was done by using SPSS (Statistical Package for Social Sciences) version 17.0 and Microsoft Excel. Chi-square test was used to assess the effectiveness of smoking cessation counselling combined with bupropion in TB patients. p-value <0.05 was considered significant.
Results
Demographic Profile
A total of 76 subjects presenting to Respiratory OPD or admitted to this centre for treatment of TB met the inclusion criteria for the study. Thirteen subjects were excluded from statistical analysis. A total of 63 subjects were eligible for statistical analysis. Their demographic profile is depicted in [Table/Fig-1]. Pharmacotherapy was stopped in 3 patients due to adverse effects out of which one patient had seizures, second patient had headache and the third had severe gastrointestinal upset. Seven patients could not be followed up and one died (due to unrelated cause) during the course of the study. Two patients were detected to have alcohol dependence syndrome. Smoking cessation interventions were stopped in these patients and they were started on de-addiction therapy by Psychiatrist for alcohol dependence. There were 29 subjects in <35 years age group and 34 subjects in ≥35 years age group. There were 3 (4.7%) patients suffering from Hypertension, Human Immunodeficiency Virus (HIV) infection and airway disease each in the study population. One (1.5%) patient was a case of Non-insulin dependent diabetes mellitus.
Demographic profile of study population (n=63).
| Variable | Frequency | Percentage |
|---|
| Age groups (years) |
| <35 | 29 | 46.0% |
| ≥35 | 34 | 54.0% |
| Gender |
| Male | 63 | 100.0% |
| Marital status |
| Married | 44 | 69.8% |
| Single | 19 | 30.2% |
| Admission status |
| Admitted | 46 | 73.0% |
| OPD | 17 | 27.0% |
| Sputum status |
| Positive | 15 | 23.8% |
| Negative | 48 | 76.2% |
Nicotine Dependence
There were 48 (76.2%) patients with high nicotine dependence, followed by 8 (12.7%) with moderate nicotine dependence and 7 (11.1%) with low nicotine dependence. Age-wise distribution of nicotine dependence in study subjects showed low nicotine dependence in 3 (10.3%) patients in <35 years age group and 4 (11.7%) patients in ≥35 years age group. There were 3 (10.3%) patients in <35 years age group and 5 (14.7%) patients in ≥35 years age group having moderate nicotine dependence. Similarly, high nicotine dependence level was seen in 23 (79.3%) patients in <35 years age group and 25 (73.5%) patients in ≥35 years age group. Difference in rates of nicotine dependence between the two age groups was not found to be statistically significant.
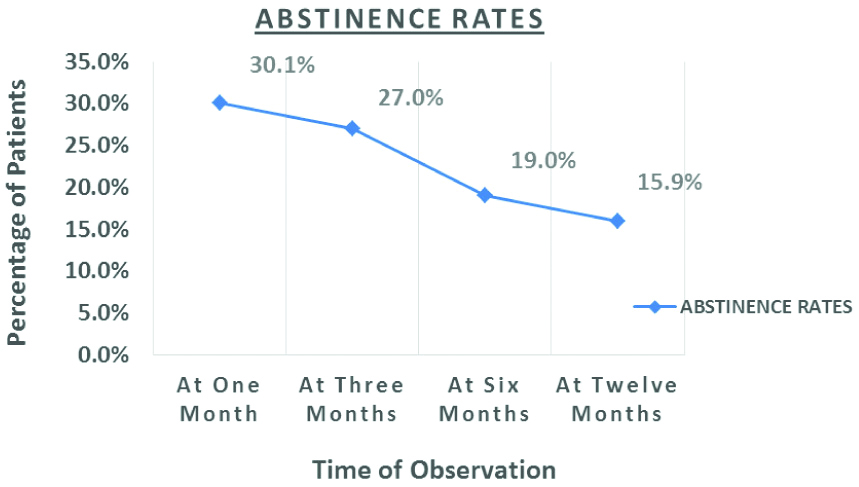
Abstinence Rates
Abstinence rates were measured at 1, 3, 6 and 12 months following start of bupropion and smoking cessation counselling [Table/Fig-2]. Abstinence rates were 19 (30.1%) patients on follow-up after one month. Abstinence rates had fallen to 17 (27%) on follow-up after three months, followed by a further fall to 12 (19%) at six months and 10 (15.9%) at 12 months.
Abstinence rates in study subjects.
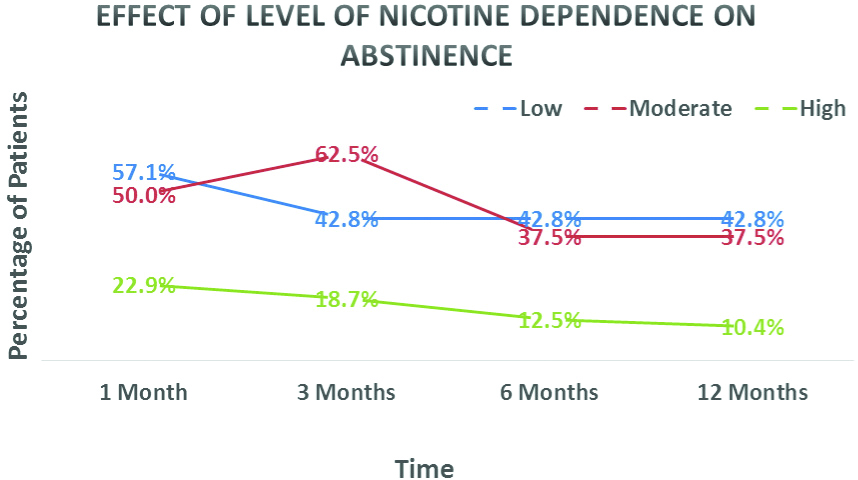
Effect of Nicotine Dependence on Outcomes
Effect of low, moderate and high nicotine dependence on abstinence rates are shown in [Table/Fig-3]. Patients with low nicotine dependence were found to have better abstinence rates at 1, 3, 6 and 12 months of follow-up and differences between the groups were statistically significant. In patients with high nicotine dependence 12 (25.0%) were sputum positive, followed by 2 (25.0%) in patients with moderate nicotine dependence and 1 (14.2%) in patients with low nicotine dependence. The difference in incidence of sputum positivity between high and low levels of nicotine dependence was statistically significant.
Effect of nicotine dependence on outcomes in study subjects.
Adverse Effects
The commonest side effect was dry mouth/altered taste in 9 (14.3%) patients, followed by gastrointestinal upset in 8 (12.7%) patients, headache in 7 (11.1%) patients, insomnia in 5 (7.9%) patients, abnormal dreams in 3 (4.7%) patients and anxiety in 2 (3.2%) patients.
Discussion
Since all study subjects were young, patients in age group <45 years predominate among study subjects. All patients in the study were males, though female sex was not an exclusion criterion. 44 (69.8%) patients amongst study subjects were married. Pressure from spouse and children may have influenced the patient’s decision to quit smoking. 48 (76.2%) patients were sputum negative for acid fast bacilli while 15 (23.8%) patients were sputum positive for acid fast bacilli at the outset of the study. Smear negative patients were diagnosed on clinic-radiological basis. This is borne out by a similar study which showed higher rates of sputum positivity in TB patients, who were smokers [17].
Nicotine dependence of patients was assessed using FTND. There were 48 (76.2%) patients with high nicotine dependence, followed by 8 (12.7%) with moderate nicotine dependence and 7 (11.1%) with low nicotine dependence. The high rates of high nicotine dependence were due to the fact that patients who continue to smoke during a major illness are likely to have high nicotine dependence. Bupropion induced seizures have been rarely reported in drug safety surveillance studies and case reports at a rate of 1 in 1000 patients [18,19].
In the present study, abstinence rates were 19 (30.1%) patients on follow-up after one month. Abstinence rates had fallen to 17 (27%) on follow-up after three months, followed by a further fall to 12 (19%) at six months and 10 (15.9%) at twelve months. With time abstinence rates are bound to fall as the subjects relapse back into smoking. As per a systemic review by Whitehouse E et al., most smoking cessation interventional studies utilizsed brief physician/healthcare worker advice to quit [20]. In a study by Siddiqi K et al., tuberculosis patients were randomizsed into control, BSS (Behavioural support sessions) and BSS with Bupropion. Abstinence rates were 8.5% in controls, 41% in BSS group and 41.5% in BSS with Bupropion group at six months of follow-up. This study concluded that there was no added benefit of Bupropion over smoking cessation counselling. However, this study recruited all patients who were suspected to have tuberculosis, unlike the present study where only confirmed pulmonary TB cases on ATT were recruited. Bupropion dose administered in this study was 150 mg/day, while we used 150 mg Bupropion twice a day in the present study, leading to better effectiveness of Bupropion [21]. Another study by TS Bam which studied effectiveness of NRT with monthly smoking cessation counselling showed abstinence rates of 41.1% at one month of follow-up and 66.8% at six months of follow-up. However, this study used self-reported abstinence for determining abstinence, which is prone to pitfalls. Secondly, they included all patients who smoked even a puff in last three months before recruitment into the study as current smokers, while the present study had a cut-off of one month for current smokers. These factors may have led to lower abstinence rates in the present study [6,22,23]. The lower abstinence rates in the present study are a reflection of the fact that a significant chunk of TB patients stop smoking on their own, due to disease or general debility and are not prescribed smoking cessation medications. Thus, TB patients enrolled in the present study, who continued to smoke despite suffering from a major illness, were more likely to have higher nicotine dependence. These patients were less likely to stop smoking at the outset and less likely to remain abstinent during the course of the study.
Effects of level of nicotine dependence on abstinence rates, was also studied. Patients with low nicotine dependence were found to have better abstinence rates at 1, 3, 6 and 12 months of follow-up and differences between the groups were statistically significant. Similar results were obtained in another study where brief interventions were not successful in patients with high nicotine dependence [24].
The present study also found a positive correlation between level of nicotine dependence and sputum positivity in the study subjects. The difference in incidence of sputum positivity between various levels of nicotine dependence was statistically significant. A study conducted in Japan in 2012 showed increased sputum positivity in heavy smokers [25]. The frequency of adverse effects in these studies, largely mirror the adverse effects seen in the present study [22].
limitation And Future Recommendations
Due to design constraints effect of smoking cessation interventions on TB outcomes have not been studied and subjects have not been followed up beyond one year. Larger studies involving multiple DOTS centres should be done in India to study changes in TB outcomes with smoking cessation interventions. Long term follow-up studies can also be done to study relapse rates in TB patients treated with smoking cessation interventions.
Conclusion
TB and tobacco smoking put a great strain on healthcare resources and usually coexist in developing countries. TB patients who continue to smoke should be offered smoking cessation counselling and pharmacotherapy. Such patients are likely to be more receptive to smoking cessation interventions, which will, in turn, lead to better outcomes for smoking cessation as well as TB.
Abstinence rates with bupropion and smoking cessation counselling at 12 months of follow-up were found to be 15.9%, which were lower than outcomes in other similar studies. Abstinence rates were found to be higher in patients with low nicotine dependence. Larger population-based studies on smoking cessation interventions need to be conducted in Indian TB patients who smoke tobacco. The study will provide data on smoking cessation with counselling and bupropion in Indian patients suffering from TB.
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. Yes
Plagiarism Checking Methods: [Jain H et al.]
Plagiarism X-checker: Aug 08, 2019
Manual Googling: Sep 12, 2019
iThenticate Software: Oct 07, 2019 (9%)
[1]. WHO. Media centre fact sheet on smoking statistics May 2019 2019 [cited 2019 08 July]. Available from: https://www.who.int/news-room/fact-sheets/detail/tobacco [Google Scholar]
[2]. WHO. Global adult tobacco survey (GATS 2) 2016-17 [cited 2019 9 July]. Available from: https://www.who.int/tobacco/surveillance/survey/gats/GATS_India_2016-17_FactSheet.pdf [Google Scholar]
[3]. Gupta PC, The public health impact of tobaccoCurrent Science 2001 5:475-81. [Google Scholar]
[4]. Peto R, Smoking and death: the past 40 years and the next 40BMJ 1994 309(6959):937-39.10.1136/bmj.309.6959.9377950669 [Google Scholar] [CrossRef] [PubMed]
[5]. Silagy C, Physician advice for smoking cessationCochrane Database Syst Rev2000(2):Cd00016510.1002/14651858.CD000165 [Google Scholar] [CrossRef]
[6]. Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, A comparison of sustained-release bupropion and placebo for smoking cessationN Engl J Med 1997 337(17):1195-202.10.1056/NEJM1997102333717039337378 [Google Scholar] [CrossRef] [PubMed]
[7]. Crocq MA, Alcohol, nicotine, caffeine, and mental disordersDialogues in Clinical Neuroscience 2003 5(2):175-85. [Google Scholar]
[8]. Smith PH, Homish GG, Giovino GA, Kozlowski LT, Cigarette smoking and mental illness: a study of nicotine withdrawalAmerican Journal of Public Health 2014 104(2):e127-33.10.2105/AJPH.2013.30150224328637 [Google Scholar] [CrossRef] [PubMed]
[9]. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summaryRespiratory Care 2008 53(9):1217-22. [Google Scholar]
[10]. Behera D, Uppal R, Majumdar S, Urinary levels of nicotine & cotinine in tobacco usersThe Indian Journal of Medical Research 2003 118:129-33. [Google Scholar]
[11]. Kim S, Overview of Cotinine Cutoff Values for Smoking Status ClassificationInternational Journal of Environmental Research and Public Health 2016 13(12)10.3390/ijerph1312123627983665 [Google Scholar] [CrossRef] [PubMed]
[12]. WHO. Global tuberculosis report 2018 [cited 2019 9 July]. Available from: https://www.who.int/tb/publications/factsheet_global.pdf?ua=1 [Google Scholar]
[13]. WHO. Tuberculosis country profiles 2017 [cited 2019 9 July]. Available from: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=IN&LAN=EN&outtype=html [Google Scholar]
[14]. Tobacco smoking and tuberculosis. Training module for medical practitioners: Revised National Tuberculosis Control Programme (RNTCP) 2010;50-51 [Google Scholar]
[15]. Wang MG, Huang WW, Wang Y, Zhang YX, Zhang MM, Wu SQ, Association between tobacco smoking and drug-resistant tuberculosisInfection and Drug Resistance 2018 11:873-87.10.2147/IDR.S16459629928135 [Google Scholar] [CrossRef] [PubMed]
[16]. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO, The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance QuestionnaireBritish Journal of Addiction 1991 86(9):1119-27.10.1111/j.1360-0443.1991.tb01879.x1932883 [Google Scholar] [CrossRef] [PubMed]
[17]. Agrawal VK, Agarwal A, Impact of tobacco smoke on tuberculosis: a case control studyNJIRM 2011 2(3):38-42. [Google Scholar]
[18]. Hill S, Sikand H, Lee J, A case report of seizure induced by bupropion nasal insufflationPrimary Care Companion to the Journal of Clinical Psychiatry 2007 9(1):67-69.10.4088/PCC.v09n0114a17599174 [Google Scholar] [CrossRef] [PubMed]
[19]. Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T, Antidepressants for smoking cessationCochrane Database Syst Rev 2014 1:Cd00003110.1002/14651858.CD000031.pub4 [Google Scholar] [CrossRef]
[20]. Whitehouse E, Lai J, Golub JE, Farley JE, A systematic review of the effectiveness of smoking cessation interventions among patients with tuberculosisPublic Health Action 2018 8(2):37-49.10.5588/pha.18.000629946519 [Google Scholar] [CrossRef] [PubMed]
[21]. Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trialAnnals of Internal Medicine 2013 158(9):667-75.10.7326/0003-4819-158-9-201305070-0000623648948 [Google Scholar] [CrossRef] [PubMed]
[22]. Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessationN Engl J Med 1999 340(9):685-91.10.1056/NEJM19990304340090310053177 [Google Scholar] [CrossRef] [PubMed]
[23]. Kumar R, Kushwah AS, Mahakud GC, Prakash S, Smoking cessation interventions and continuous abstinence rate at one yearIndian J Chest Dis Allied Sci 2007 49(4):201-07. [Google Scholar]
[24]. Horn K, Fernandes A, Dino G, Massey CJ, Kalsekar I, Adolescent nicotine dependence and smoking cessation outcomesAddictive Behaviors 2003 28(4):769-76.10.1016/S0306-4603(02)00229-0 [Google Scholar] [CrossRef]
[25]. Matsumoto K, Arima K, Komukai J, Danno K, Yoshida H, Hirota S, The association between smoking and sputum smear-positive pulmonary tuberculosis in Osaka CityKekkaku: [Tuberculosis] 2012 87(8):541-47. [Google Scholar]