Neonatal Cholestasis Syndrome (NCS) is defined as prolonged elevation of conjugated bilirubin of >1 mg/dL if Total Serum Bilirubin (TSB) is <5 mg/dL, or conjugated bilirubin of >20% TSB if >5 mg/dL, beyond 14 days of age in a neonate [1]. Paediatricians are aware of the various aetiologies of NCS and its major categorisation into Extrahepatic (mainly BA) and Intrahepatic (mainly Idiopathic hepatitis). Yet there are delayed referrals which in turn increase the chance of liver failure. Studies on aetiological spectrum [Table/Fig-1] have quoted around 40-50% of NCS to be caused by BA and around 25-30% to be Idiopathic neonatal hepatitis [2-7]. Most of these studies have been done in smaller cohort of 30-100 infants. In the last decade with the expansion of genetic facilities and availability of various metabolic tests, more patients categorised to have Idiopathic hepatitis are diagnosed to have a specific cause. This is highlighted to change in spectrum of NCS [8] with BA contributing only 25% of NCS and other metabolic and genetic disorders being identified as an intrahepatic aetiology. Surprisingly, the true outcome of the “Idiopathic neonatal hepatitis” group has not been outlined by any study.
The present study thus proposes to analyse aetiological basis of NCS amongst a large cohort of infants. Assessment of outcome of Intrahepatic specially the Idiopathic hepatitis group in terms of liver function and growth is also proposed to be studied. Hepatobiliary scan (HBS) in diagnosing BA is the single most important timed screening investigation if a surgical cause is suspected. In this study, the sensitivity of HBS in screening for BA will also be analysed.
Recognising the need of early diagnosis, many countries such as the UK have launched “The Yellow Alert Campaign”. Also, in Taiwan introduction of stool colour card system resulted in early referrals of infants with NCS [9]. Thus, awareness of aetiological spectrum and knowledge of HBS is of utmost importance while dealing with these infants. This may also help establish early warning signs and referral guidelines in our country. The present study thus outlined the aetiological spectrum of infants with NCS and studies the clinical outcome.
Materials and Methods
This was a retrospective observational study done from Jan 2008-Dec 2018. Data was obtained from the online clinical portal and the HBS register. All infants with diagnosis of NCS seen in the outpatient service or having been admitted in the ward were included in the study. Clinical outcome was assessed in non-surgical patients with a minimum of 6 month follow-up period. All those who presented post-surgery were excluded. The proposal (CMC IRB No 11557 dated 1.10.2018) was accepted by the Institutional review board and Ethics Committee.
As per standard practice, all infants who fulfil the diagnostic criteria for NCS undergo full liver function test and ultrasound abdomen. Depending on the history and clinical features the following tests are done in most patients- serum amino acid, serum citrulline, Alpha fetoprotein (AFP), Toxoplasmosis Rubella Cytomegalovirus Herpes simplex infections (TORCH) IgM, blood culture, thyroid function, Urine reducing substance and Urine chromatography for galactose. Next tier of investigations such as Cytomegalovirus (CMV) PCR, Ferritin, Hemophago-lymphohistiocytosis (HLH) work-up, Gal-1-P levels, Urinary succinyl acetone, Karyotyping, Genetic testing are done only in select few based on clinicians’ decision and affordability of the patient; very similar to guidelines [10]. Liver biopsy is done in most, but in the recent past, there has been a downward trend as more emphasis is being laid on extensive non-invasive work-up inclusive of clinical exome analysis.
All patients are treated with multivitamin therapy with A,D,E,K, Ursodeoxycholic Acid (UDCA) and medium chain triglyceride as per guidelines [11].
For the purpose of the study, the various aetiologies were diagnosed based on following criteria:
Sepsis- Blood culture positive cases only
Intra-uterine (IU) infection:
CMV- if urine or blood PCR positive (only CMV IgM positive cases excluded)
Toxoplasmosis- Toxoplasma IgM positive with retinal changes considered positive
Metabolic disorders
Galactosemia- Urine chromatography positive for Galactose or Dbs showing Galactose-1-P > 20 mg/dL
Tyrosinemia- Serum Tyrosine level >3 times upper limit of normal for age or urinary succinyl acetone positive or genetic test positive
Mitochondrial hepatopathy- genetic test positive cases only
Neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD)- Elevated serum Citrulline levels (HPLC)
Hyper-ornithenemia- Elevated serum Ornithine levels (HPLC)
Genetic disorders
Allagille syndrome- Bile duct paucity on liver biopsy and/or 3/5 major clinical criteria positive (as per Gene reviews)
Down- Karyotyping suggestive of Down’s syndrome
Dubin Johnson- genetic test positive only
Arthrogryposis Renal tubulopathy Cholestasis syndrome (ARC)- clinical or genetic test positive
Unspecified- if genetic test not done
PFIC- based on liver biopsy and Immunohistochemistry (IHC) pattern (CD 13 for PFIC1, BSEP for PFIC2, MDR3 for PFIC3)
Caroli- Based on ultrasound findings
Langerhan Cell Histiocytosis (LCH)- based on liver biopsy and Immunohistochemistry pattern
HLH- based on bone marrow findings and biochemical parameters and/or genetic positive
Results
Total of 300 infants (210 males) were included in the study. A total of 232 patients were identified as intra-hepatic group. Baseline demography was similar in the 2 groups as shown in [Table/Fig-2].
Baseline demography among the 2 aetiological groups.
| Parameter | Intrahepatic (n=232) | Extrahepatic (n=68) | p-value |
|---|
| Birth weight in Kg (mean±SD) | 2.58±0.59 | 2.52±0.65 | 0.543* |
| Term gestation | 191 (82.7%) | 56 (82.4%) | 0.862# |
| Male | 166 (71.6%) | 44 (64.7%) | 0.298# |
p-value <0.05 significant, *T-test, #Chi-square test
Of the 232, 5 patients died, 5 were referred for liver transplant and 2 got discharged against medical advice, 110 had less than 4 month of follow-up and were hence not studied. Outcome analysis was thus done in 95/232 i.e., 41% of the cohort [Table/Fig-3]. In the cohort of 300- all had history of jaundice with high coloured urine. All underwent blood tests and ultrasound abdomen. A total of 72/300 (24%) underwent liver biopsy- giant cell transformation with cholestasis was seen in 35 indicating Neonatal hepatitis (2 of whom had cirrhosis noted on liver biopsy), PFIC was diagnosed in 11 (based on typical immunohistochemistry pattern) and bile duct paucity in one. BA was reported in 24.
Aetiological spectrum of NCS.
| Aetiology | Number (n=300) | Total (100%) |
|---|
| Intrahepatic/Non-surgical (N=232) |
| Idiopathic | 164 | 54.6 % |
| Infection-sepsis$ | 9 | 3 % |
| Congenital Intrauterine Infections | 4 | 1.3% |
| Metabolic# | 22 | 7.3% |
| Genetic* | 14 | 4.7 % |
| Bile duct disorders^ | 13 | 4.3% |
| TPN induced Neonatal hepatitis | 3 | 1% |
| Others (LCH-1, HLH-2) | 3 | 1 % |
| EXTRAHEPATIC/OBSTRUCTIVE (n=68) |
| BA | 64 | 21.3% |
| Choledochal cyst | 2 | 0.7 % |
| Malrotation with midgut volvulus causing obstructive jaundice | 1 | 0.3% |
| Inspissated bile duct syndrome | 1 | 0.3% |
$ Sepsis- E Coli (3), Klebsiella (3), Burkholderiacepaciae (1), Enetrobacter Cloace (1), Non Fermenting Gram Negative Bacilli (1); #Metabolic- Tyrosinemia (8), Galactosemia (5), Storage disorders (2), hypothyroidism (2), unclassified (2), NICCD (1), Hyperornithenemia (1), Mitochondrial (1); *Genetic- Allagille (3), Down syndrome (3), Peroxisomal storage disorder (1), ARC syndrome (1), Congenital conjugated hyperbilirubinemia (2), Down syndrome (2), unclassified (2); ^Bile duct disorders- Progressive Familial Intrahepatic Cholestasis PFIC (11), bile duct paucity (1), Caroli disease (1); TPN: Total parenteral nutrition
The mean age at presentation was 2.4 months in the intrahepatic group and 3 months in the Extrahepatic group.
At presentation, weight was 3.8 kg in the intrahepatic group, and 4.4 kg in the BA which was statistically significant (p- 0.005).
Aetiological spectrum- In total, 232 (77%) had intrahepatic and 68 (23%) had extrahepatic cause of NCS. Aetiological distribution has been represented in [Table/Fig-4].
NCS follow-up flow chart.
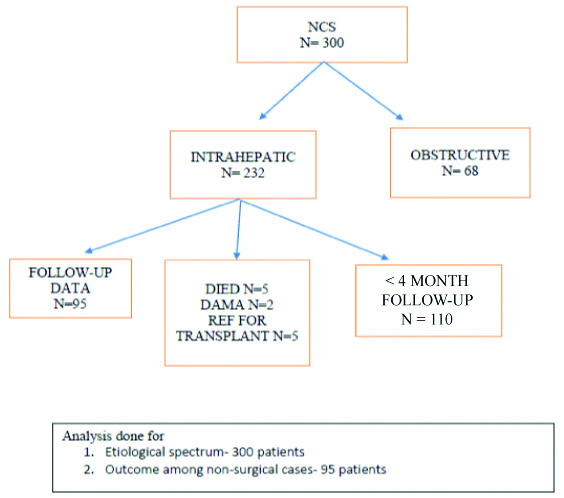
Medical records also showed that 5 succumbed to the illness (1 Tyrosinemia, 1 mitochondrial disease, 3 idiopathic with decompensated liver disease at presentation), 2 got discharged against medical advice (1 Tyrosinemia, 1 storage disorder) and 5 with cirrhosis were referred for liver transplant.
Outcome analysis- this was done in 95 infants, at a mean follow-up of 23.5 months (range 6-96 months).
There was significant improvement in weight centile noted at follow-up. Height percentile at follow-up also was higher than at onset. When considering only the Idiopathic group, the improvement in height (mean 29.8 centile) was even better than the whole cohort, indicative of good long-term growth potential [Table/Fig-5].
Growth and Biochemical outcome at follow-up (n=95).
| At presentation (mean 2.4 months of age) N=232 | At last follow-up (mean 23.5 months of age) N=95 | p-value |
|---|
| Weight centile | 8.9 | 27.7 | 0.00 |
| Height centile | 16.7 | 22.4 | 0.098 |
| At presentation | At last follow-up | p-value |
| Total bilirubin (mg/dL) | 11.6 | 0.43 | 0.00 |
| Direct bilirubin (mg/dL) | 7.27 | 0.19 | 0.00 |
| SGOT (IU/L) | 113.9 | 52.9 | 0.00 |
| SGPT (IU/L) | 256.6 | 40.3 | 0.00 |
| Albumin (gm/dL) | 3.7 | 4.5 | 0.00 |
| ALP (IU/L) | 538.6 | 293.5 | 0.00 |
| AFP | 56181.9 | 14.2 | 0.00 |
*p-value <0.05 significant, paired t-test
There was also a significant improvement in all liver function parameters with near normalisation at follow-up [Table/Fig-5], indicative of good liver function.
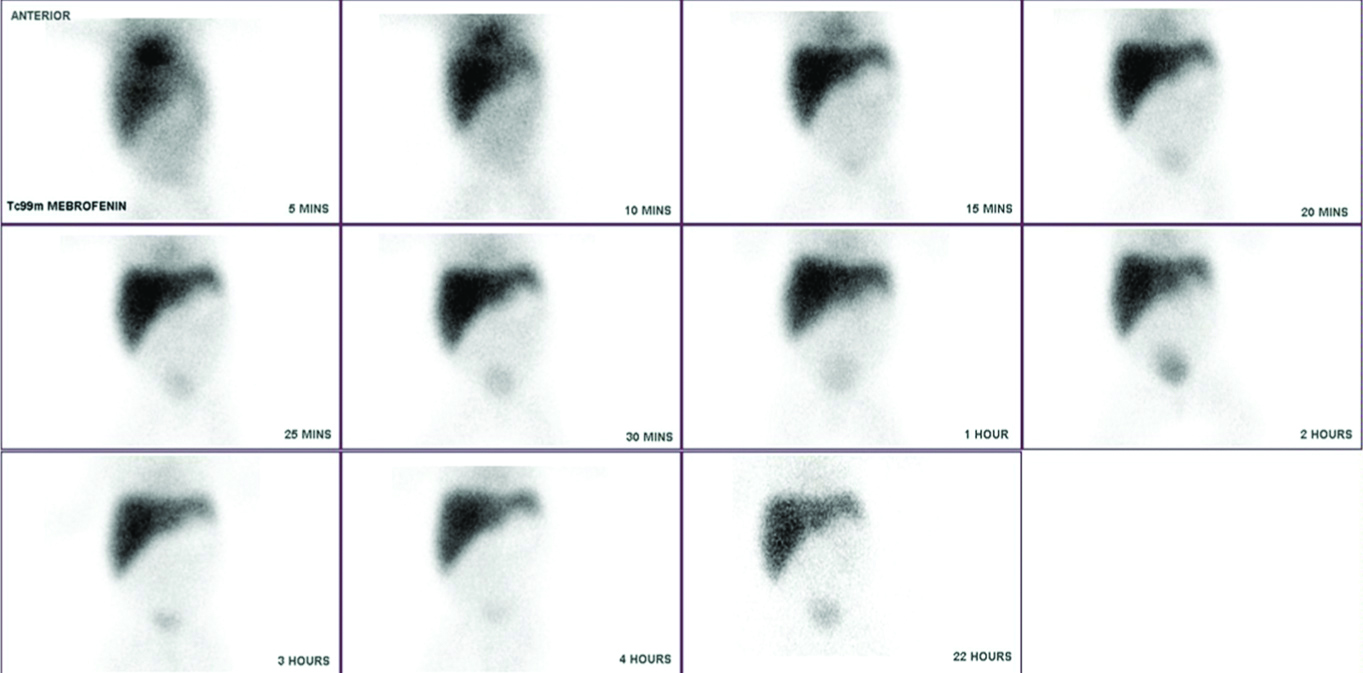
Hepato-biliary Scan (HBS) analysis was done in 68 NCS cases in the intra-hepatic group. The typical finding of BA is shown in [Table/Fig-6]. A 2×2 table comparing the gold standard of cholangiogram and HBS is shown in [Table/Fig-7]. Overall HBS scan had a good sensitivity of 90% and a very poor specificity of 5.6 % for diagnosis of BA.
HBS image of a neonate with BA showing no excretion into the bile duct at 22 hour image.
Comparison of HBS to intra-operative cholangiogram for diagnosis of Biliary atresia (n=68).
| Intra-op cholangiogram s/o BA Pos | Neg |
|---|
| HBS suggestive of BA Pos | 45 | 17 |
| Neg | 5 | 1 |
Discussion
This study was done on 300 infants with NCS. Majority (70%) were boys. The mean age at presentation of 2.4-3 months was similar to previous studies on neonatal cholestasis in India, Bangladesh and Pakistan [1,2,4].
At birth, the weight of neonates was similar in both groups as noted in [Table/Fig-2]. However, at presentation, the weights were statistically different highlighting that infants with intra-hepatic NCS demonstrate poor weight gain from early infancy.
Of the 300 infants, 17% were born preterm and 83% term. The distribution of term: preterm was similar in both groups [Table/Fig-2]. This is distinctly different from the cohort of 30 infants with NCS where 92% of infants with BA were term, whereas the intra-hepatic group had a predominant 56% as pre-term [5]. The present study may be a true representation of this data as a larger cohort was analysed.
In this study, BA constituted only 21.3% of the cohort. This is again very distinct from literature published so far which overbearingly shows BA constituting 42.5% [6] to 55% [12] of NCS.
The most common cause of NCS was Idiopathic neonatal hepatitis seen in 55%. This study showed a wider distribution of aetiologies probably because of the facility of metabolic tests, genetic testing and liver biopsy available in-house. Intra-uterine infections constituted 1.3%. Only 1 previous study showed IU infection as aetiology for NCS [5]. In another study from Central India, sepsis was identified in 14% and no report of IU infection was made [7]. Thus, the index study highlights that congenital infections are still prevalent and need to be included in the aetiological work-up. Genetic analysis has improved the diagnostic data and there is only report of one study [6] quoting genetic syndromes [Table/Fig-1].
Total 68 infants (22.7%) in study cohort had extra-hepatic cause of neonatal cholestasis among which BA constituted majority. Causes other than BA were also identified as enlisted in [Table/Fig-4]. This has also not been reported in previous studies.
Clinical outcome for the identified aetiologies among the intrahepatic NCS at follow-up was studied. This included the growth (length and weight centile), liver function tests including total and direct bilirubin levels, liver enzymes, alkaline phosphatase, PT with INR and albumin. A total of 5 children had died, 5 were referred for liver transplant and 2 got discharged against medical advice. Follow-up data after 6 months was available for 95 infants, i.e., 41% of the original cohort.
Clinical outcome was good with significant improvement in weight and gain in height centile. Biochemical parameters showed significant improvement at follow-up [Table/Fig-5] with near normalisation. Follow-up period was good at nearly 2 years mean with longest follow-up to 7.5 years of age noted. This has not been studied earlier.
HBS is widely used for diagnosis of BA. This study also looked at the sensitivity and specificity of HBS in diagnosis of BA [Table/Fig-7]. This study demonstrated that HBS had a sensitivity of 90% and a specificity of only 5.6% for the diagnosis of BA, as against a gold standard of intraoperative cholangiogram. The sensitivity of HBS scan fared well as compared to studies published earlier which show a sensitivity of 97-100%. However, the specificity was much lower than 33-91% quoted by earlier studies [13,14]. It is safe to conclude that HBS is a good screening test while intraoperative cholangiogram remains the gold standard for diagnosis of BA.
Limitation
This was a retrospective study and thus all details were obtained from medical records and could not be further verified. Availability of tests and affordability of patients would have played a role in degree to which the evaluation was performed.
Strengths
This is the largest cohort of patients with NCS that have been studied from Asia, to the best of our knowledge.
This study is the only one to highlight outcome analysis at follow-up and thus adds immensely to the knowledge of NCS. A good follow-up mean period of 2 years was noted with longest of upto 7.5 years of age.
As our centre is a referral centre in South India and caters to a large number of patients from India’s eastern states, our data is fairly representative of true Indian population data on NCS.
Comprehensive aetiological analysis including specific IU infections, metabolic and genetic cause has been highlighted by this study.
Availability of HBS in-house helped in studying its sensitivity in diagnosing BA.
Conclusion
This study establishes that Intra-hepatic NCS is much more common as compared to surgical causes with Idiopathic hepatitis constituting 55% of the cohort. Other causes such as ARC syndrome, mitochondrial hepatopathy, peroxisomal storage disorder, NICCD, PFIC, Caroli are being described for the first time as an aetiological diagnosis. HBS remains a good screening tool, while intraoperative cholangiogram is the gold standard for diagnosis of BA. If diagnosed and treated early, infants with intra-hepatic NCS have done well at follow-up in terms of growth and liver function irrespective of the aetiology. More studies on outcome analysis will need to substantiate this further as our follow-up of 2 year mean was noted among 41% of the cohort only.
p-value <0.05 significant, *T-test, #Chi-square test
$ Sepsis- E Coli (3), Klebsiella (3), Burkholderiacepaciae (1), Enetrobacter Cloace (1), Non Fermenting Gram Negative Bacilli (1); #Metabolic- Tyrosinemia (8), Galactosemia (5), Storage disorders (2), hypothyroidism (2), unclassified (2), NICCD (1), Hyperornithenemia (1), Mitochondrial (1); *Genetic- Allagille (3), Down syndrome (3), Peroxisomal storage disorder (1), ARC syndrome (1), Congenital conjugated hyperbilirubinemia (2), Down syndrome (2), unclassified (2); ^Bile duct disorders- Progressive Familial Intrahepatic Cholestasis PFIC (11), bile duct paucity (1), Caroli disease (1); TPN: Total parenteral nutrition