Total Anomalous Pulmonary Venous Connection (TAPVC) is a rare congenital cardiac defect, occurring in 1% to 3% of babies born with congenital cardiac defect [1]. It is almost always lethal within first month of life if no surgical intervention is done. Classification of TAPVC is based upon the site of its drainage into systemic circulation. The commonest variant is supracardiac type (45%), followed by infracardiac (25%), cardiac (25%) and mixed (5%) types [2].
TAPVC can also be classified by the presence of obstruction in the drainage. Obstruction is invariably present in the infracardiac type, whereas obstruction is rare in the cardiac type [3].
Without surgical intervention, they have a mortality which exceeds 78% in their first year [4]. Isolated TAPVC with biventricular anatomy have a better prognosis than infants with Single-ventricle (SV) physiology [5].
CT pulmonary angiography, improved surgical techniques and better post-operative management has played an important role in reducing the perioperative mortality. The study centre follows these principles. This study was done to evaluate the impact of current management strategies on surgical outcomes of TAPVC repair and occurrence of the dreaded post-repair PVO.
Materials and Methods
This prospective observational study was carried out on all patients who underwent surgical correction for isolated total anomalous pulmonary venous connection over a period of seven years at the institution. Twenty-seven patients underwent TAPVC repair between January 2012 and December 2018. These patients were recruited by consecutive enumerative sampling. This study was approved by the Ethical and Research Board and the Helsinki declaration were followed. Written informed consent in local language was obtained from the legal guardians.
The inclusion criteria were diagnosis of isolated total anomalous pulmonary venous connection. Patients with single ventricular physiology and other associated cardiac anomalies (except atrial septal defect, patent ductus arteriosus) were excluded from the study.
A pre-operative trans-thoracic echocardiogram was performed in all the patients. During echocardiographic assessment, the suprasternal long axis and its rotational views were used for detecting supracardiac TAPVC. Coronary sinus TAPVC was diagnosed over the parasternal long-axis view and in its anterolateral tilting view. In newborns with TAPVC to the coronary sinus, the pulmonary venous confluence was viewed over the apical four-chamber view and its drainage can be seen in antero-medial tilting view. Subcostal view is used to detect the course of pulmonary venous chamber drainage and its flow pattern in patients with infra-cardiac type TAPVC [6].
Computed Tomography (CT) pulmonary angiography was done in 23 patients using 128-slice Multi detector CT scanner to evaluate the anatomic features and classify TAPVC variants [Table/Fig-1].
CT pulmonary angiogram depicting left upper (LUPV), Left lower (LLPV), Right upper (RUPV) and Right Lower Pulmonary Veins (RLPV) draining into a common channel situated posterosuperior to the left atrium. This common channel opens into the right atrium just below the opening of Superior Vena Cava (SVC) into the right atrium (Cavo-atrial junction).
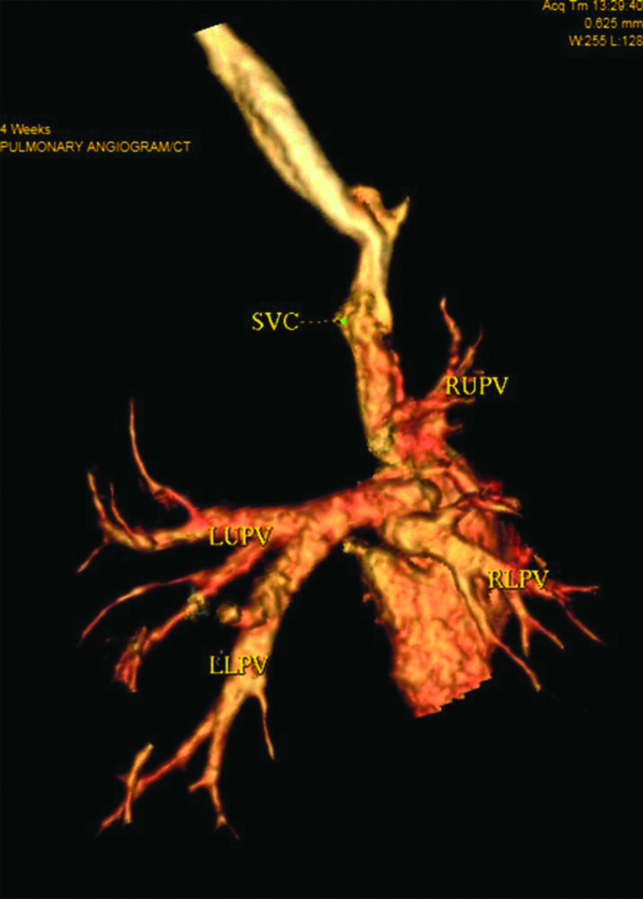
A detailed proforma was filled for all patients. This included clinical assessment, echocardiography and CT pulmonary angiography findings. Intraoperative findings, choice of procedure, cardio-pulmonary bypass time and aortic cross clamp time were documented.
An initial pre-anaesthetic assessment was done for all study participants according to American Statistical Association (ASA).
Surgical Technique
TAPVC correction was performed through median sternotomy. Aorto-bicaval Cardio-pulmonary Bypass (CPB) was established. The ductus arteriosus was identified and ligated after establishing CPB. A dose of cold antegrade blood cardioplegia was administered (30 mL/kg). Low flow cardio-pulmonary bypass with deep hypothermia was used for infracardiac and mixed variants of TAPVC.
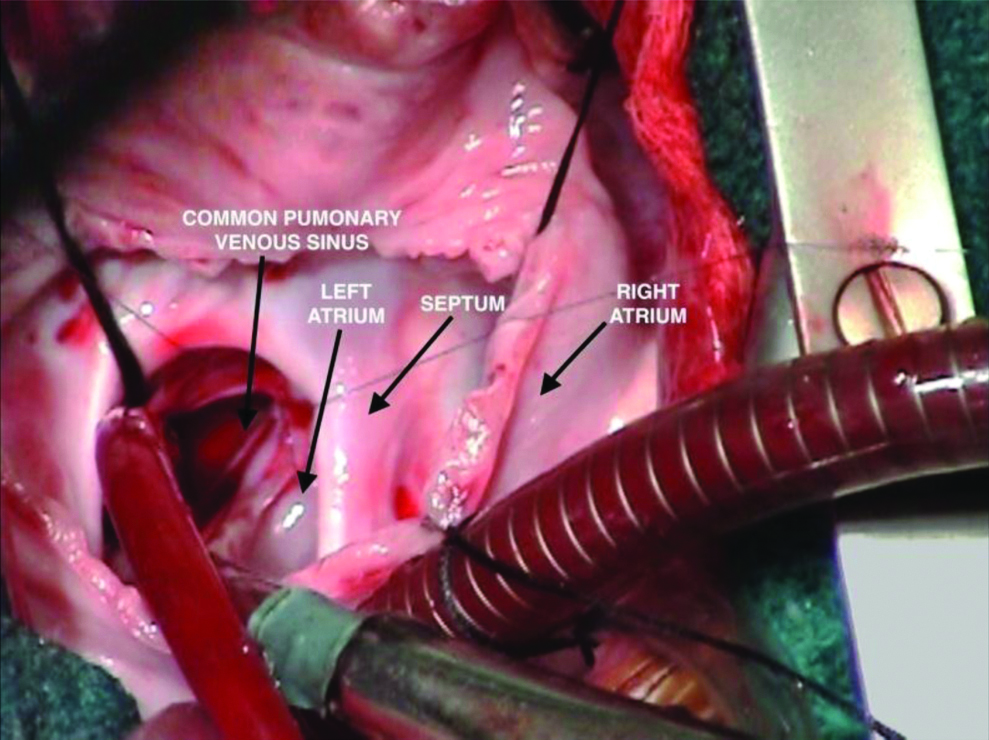
For supracardiac connections, the vertical vein were ligated outside the pericardium at the level of innominate vein. Right atrium was opened parallel to the atrio-ventricular groove. Foramen ovale membrane was excised. Posterior wall of left atrium was incised transversely into the pericardial space behind it [Table/Fig-2]. A long incision was then made in the common pulmonary venous sinus. Common pulmonary venous sinus was then anastomosed to left atrium using continuous No. 7-0 polypropylene suture. Foramen ovale was then closed with pericardial patch.
Repair of Total Anomalous Pulmonary Venous Communication to left innominate vein, Right atrial approach. Posterior wall of left atrium is incised transversely into the pericardial space behind it. A long incision is made in the common pulmonary venous sinus.
For cardiac connections, the foramen ovale membrane was excised and unroofing of coronary sinus was done. Baffle connection of the coronary sinus to the large defect was done with a pericardial patch.
For infracardiac connections, the descending vertical vein was identified by rotating the heart superiorly. Posterior wall of left atrium was incised beginning at base of the appendage. A long incision was made in the common pulmonary venous sinus. Anastomosis of common pulmonary venous sinus to left atrium was constructed using No. 7-0 polypropylene suture. Pulmonary venous sinus was then divided below the anastomosis at the level of diaphragm.
The technique of repair in mixed-type TAPVC was based upon anatomy of the particular lesion. It involved a combination of the above mentioned surgical techniques.
Follow-Up
All surgical patients who were discharged alive from hospital were followed-up in the out-patient clinics at 1 month, 3 months and 12 months. When patients did not report for follow-up, their legal guardians were contacted through telephone.
Statistical Analysis
Data were analysed with inferential and descriptive statistics. Continuous or interval based variables were expressed as mean±standard deviation. Categorical variables were analysed with chi-square test. The results were analysed using chi-square test and p-value was determined. Statistical analysis was done using MEDCALC version 18.0 (Medcalc is a statistical software designed for biomedical science at Acalcialaan 22, 8400, Ostend, Belgium). Life table analysis of the patients under follow-up was done using IBM SPSS® Statistics version 23-software.
Results
The mean age of the 27 patients was 4.86±10.08 months and mean weight was 4.69±4.13 kg. Eleven patients (40.7%) had supra cardiac TAPVC, 8 (29.6%) patients had cardiac type of TAPVC, 4 (14.8%) patients had infra cardiac TAPVC and 4 (14.8%) patients had mixed TAPVC lesions.
Among the 27 patients, 4 patients were taken up for emergency TAPVC repair:
Patient 1 was a four-day-old male neonate weighing 3.1 kg. He was tachypneic and cyanotic. The arterial oxygen saturation was 70% with worsening metabolic acidosis. Transthoracic echocardiography demonstrated infra-cardiac TAPVC with a sluggish flow in descending pulmonary vein that connected to the portal vein. Patent ductus arteriosus showed a right to left shunting. Prostaglandin E1 was administered (0.005-0.02 μg/kg/minutes). Prostaglandin E1 dilated ductus arteriosus and ductus venosus. Decompression of the pulmonary vasculature was achieved. Diminished pulmonary venous obstruction was observed at the ductus venosus level. Haemodynamic stabilisation of the neonate was achieved prior to surgery.
Patient 2 was a six-day-old male neonate.
Patient 3 was a seven-day-old female child.
Patient 4 was three-day-old male neonate. These patients presented with worsening cyanotic metabolic acidosis, oliguria and hyperkalemia. All of them had a diagnosis of supracardiac type TAPVC with restrictive ASD. They were intubated, started on inotropic supports and were subsequently taken up for surgery.
Twenty-three patients were taken up for elective surgery. The mean cardio-pulmonary bypass time for all TAPVC types was 111.04±39.82 minutes. Mean aortic cross clamp time was 61.44±25.95 minutes and mean hospital stay of the patients was 9.11±2.08 days [Table/Fig-3].
TAPVC repair for supracardiac, cardiac, infracardiac and mixed variants: Comparison of intraoperative variables and outcome (n=27).
| TAPVC repair | Mean cardio-pulmonary bypass time (in mins) | Mean aortic cross clamp time (in mins) | Survival (Number of patients who survived)/(Total number) |
|---|
| Supracardiac (n=11) | 105.36±18.23 | 60.18±19.81 | 10/11 (1 early post-operative death due to sepsis) |
| Cardiac (n=8) | 95.62±22.34 | 43.25±23.82 | 8/8 |
| Infracardiac (n=4) | 144.5±38.44 | 76±25.62 | 1/4 (3 late deaths due to Pulmonary Venous Obstruction) |
| Mixed (n=4) | 124±17.13 | 75.25±28.27 | 4/4 |
| Total TAPVC repair (n=27) | 111.04±39.82 | 61.44±25.95 | 23/27 |
In cardiac type TAPVC, the mean cardio-pulmonary bypass time for repair was 95.62±22.34 minutes and mean cross clamp time was 43.25±23.82 minutes. In supra-cardiac TAPVC, the mean cardio-pulmonary bypass time for repair was 105.36±18.23 minutes and mean aortic cross clamp time was 60.18±19.81 minutes. In infracardiac type of TAPVC, the mean cardio-pulmonary bypass time for repair was 144.5±38.44 minutes and cross clamp time was 76±25.62 minutes. In mixed TAPVC, the mean cardio-pulmonary bypass time for repair was 124±17.13 minutes and aortic cross clamp time was 75.25±28.27 minutes. The duration of cardio-pulmonary bypass in infracardiac type of TAPVC was significantly higher than supracardiac (p-value=0.0167, CI 8.32-69.95) and cardiac variants (p-value=0.0177, CI 9.89-29.37).
Delayed sternal closure was performed in three patients to facilitate recovery of right ventricular function. Diaphragmatic plication was done in two patients. One patient with supracardiac TAPVC repair developed acute respiratory distress and was re-intubated. One patient received Extra Corporeal Membranous Oxygenation (ECMO) support, but died two days later due to bacterial sepsis.
A total of 26 patients were discharged from hospital with a mean hospital stay of 9.11±2.08 days.
All Patients were under regular follow-up. Twenty-three patients were followed for one year, one patient was followed for six months and two patients were followed for three months. Life table analysis was done. The cumulative proportion of patients surviving at the end of one year was 0.88 (standard error of 0.07) [Table/Fig-4].
Life table of the 26 patients under follow-up (median survival time is 12 months).
| Interval start time (in months) | Number of patients entering the interval | Number of patients withdrawing during the interval | Number exposed to risk of death | Number of death | Cumulative proportion of patients surviving at the end of interval | Standard error of cumulative proportion surviving at the end of interval |
|---|
| 0 months | 26 | 0 | 26.000 | 0 | 1.00 | 0.00 |
| 3 months | 26 | 0 | 26.000 | 1 | 0.96 | 0.04 |
| 6 months | 25 | 4 | 23.000 | 2 | 0.88 | 0.07 |
| 9 months | 19 | 0 | 19.000 | 0 | 0.88 | 0.07 |
| 12 months | 19 | 19 | 9.500 | 0 | 0.88 | 0.07 |
During follow-up, three patients who underwent infracardiac TAPVC repair were re-admitted with post-operative PVO. One patient was re-admitted after three months of surgery and the other two were re-admitted after six months of surgery. Re-intervention was done in two patients by pulmonary vein balloon dilatation in which one patient had mortality on operation table due to rupture of veins. The second patient tolerated procedure but died on the subsequent day. Third patient died on the day of admission despite adequate resuscitative measures [Table/Fig-3].
Among 27 patients who underwent TAPVC repair in the present institution, early death was seen in 1 (3.7%) patient and late death was seen in 3 (11.1%) of patients. No major complications were seen in 85.2% of patients.
Discussion
In this study, most of the patients underwent CT pulmonary angiogram following echocardiography. They provide a three-dimensional spatial orientation of the morphology of TAPVC variant and aid in planning the surgical correction.
Some neonates with PV obstruction often encounter systemic hypoperfusion and progressive metabolic acidosis. These infants should undergo surgical intervention at the earliest following initial haemodynamic stabilisation. In selected patients, there is role for prostaglandin E1 infusion.
Post-operative PVO was a major cause of mortality in the study. Several studies have emphasised the role of PVO as an important predictor of surgical outcome [3,7-10].
A multicentric retrospective study of 406 patients of TAPVC analysed the outcome of PVO. It showed that post-operative PVO tends to appear in the first six months after TAPVC repair and can be progressive [7]. A similar observation was made in the index study. All three patients with post-repair PVO presented within six months. This observation warrants a close follow-up of this subset of patients to enable early intervention for PVO before irreversible secondary changes occur.
Pre-operative pulmonary vein stenosis not only increases the risk of late PV restenosis, but also the overall mortality. PV restenosis remains the most important issue after correction of TAPVC [8].
Patients with infra-cardiac type TAPVC, pre-operative PVO and a longer duration of CPB have a higher likelihood of death. Stenosis of the anastomotic site and denovo stenosis of individual pulmonary veins are two principal causes of post-operative PVO [3]. Present study showed that duration of cardio-pulmonary bypass in infracardiac type of TAPVC was significantly higher than supracardiac and cardiac variants.
Padalino MA et al., presented their 22-year experience of surgical outcomes of TAPVC repair. TAPVC associated with major congenital cardiac anomaly showed a consistently high morbidity and mortality. They concluded that isolated TAPVC variant show good surgical results. Pulmonary vein obstruction is uncommon but a dangerous complication. At a median follow-up of 2.97 years (range 43 days to 22 years, 91% complete), there were nine late deaths (24.3%) [10].
Yong MS et al., presented their 36 year period study of 112 patients who underwent TAPVC repair. The patients were followed with intervals from 3 to 6 months with echocardiogram during the first 2 years after surgery, followed by annual assessment. They concluded that patients who survive beyond 2 years have an excellent outcome. Low weight at the time of surgery, pre-operative clinical status, long cardio-pulmonary bypass time, persistence of micro-obstruction were risk factors for mortality [11].
In the present study, 26 patients were followed-up, with a median follow-up duration of 12 months. Life table analysis showed an acceptable cumulative proportion of patients surviving at the end of 1 year (0.88 with standard error of 0.07).
Husain SA et al., reported that patients presenting with obstruction at the time of surgery have the highest mortality. Reintervention in patients with recurrent pulmonary venous obstruction is associated with prolonged cardio-pulmonary bypass and aortic cross-clamp times [12]. In the index study, infra-cardiac type TAPVC repair had prolonged cardio-pulmonary bypass times.
In this study, infra-cardiac TAPVC and pre-operative PVO were associated with a poorer prognosis. Post-operative PVO was seen in three of our patients. None survived. Post-operative pulmonary venous obstruction was found to be a major risk factor for mortality. This subset of patients represents the extreme end of TAPVC spectrum. They present with metabolic acidosis, hypercapnia or hypoxia. Surgical repair is complex and often requires longer cardio-pulmonary bypass support.
Overall, the surgical outcome of TAPVC repair was acceptable; 85.2% of the patients underwent TAPVC repair safely without major complications.
Limitation
The results obtained in this study need to be further substantiated in future prospective studies with a larger sample size and a longer follow-up duration.
Conclusion
Total Anomalous Pulmonary Venous Connection (TAPVC) is a surgical emergency. Acceptable outcomes after surgical correction for TAPVC can be achieved. Infracardiac TAPVC and pre-operative Pulmonary Venous Obstruction (PVO) are associated with a poorer prognosis. The duration of cardio-pulmonary bypass in infracardiac type of TAPVC is significantly higher than supracardiac and cardiac variants. This subset of patients represents the extreme end of TAPVC spectrum where they often require prolonged cardio-pulmonary bypass support.