Typhoid is a major cause of morbidity with an estimated global incidence of 22 million cases and 2 lakh deaths per year. It is a substantial public health problem in South and East Asia (>100 cases per 100,000 people annually), Africa, Latin America, and the Caribbean and Oceania (10 to 100 cases per 100,000 people annually) [1]. Typhoid fever is also the main cause of morbidity among younger children [2,3].
In some developing countries, the annual incidence may reach 1% with case-fatality rates as high as 10%, with about 70% of all deaths in Asia [4]. Typhoid is rare in industrialised nations, though travellers to endemic countries may occasionally acquire the disease [5].
In India, health surveys conducted by the Ministry of Health indicated morbidity in the range of 102-2,219/100,000 population. Bhatta CP et al., study in urban slum reported that 1% of children up to 17 yr of age suffer from typhoid fever annually [6]. Rapid rise of antibiotic resistance documented in Asia have increased the difficulty and cost of treatment; and threatens to further increase the case fatality associated with the disease.
Bacteriophages have enormous potential to be used as antibacterial, phage display systems and as a vehicle for vaccine delivery. The clinical use of phages will lead to easier treatment of the bacterial infections resistant to newer antibiotics. Many phages have been identified that act against the ‘Vi’ capsular antigen of S. Typhi. Phages could be used to deliver a protective antigen such as a DNA or a phage display vaccine. Before implementing any phage-based intervention procedure whole genome sequencing of bacteriophages is a pre-requisite. Molecular characterisation of phages may help us in better understanding of the genome organisation.
Based on limited literature of the subject, and keeping in view the growing problems associated with typhoid and the feasibility of phage therapy for this disease, the study was planned to explore the genomic structure of bacteriophages specific to ‘Vi’ positive S. Typhi.
Materials and Methods
The experimental study was carried out in the Department of Biochemistry in collaboration with the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi from February 2013 to July 2014.
Isolation and Propagation of Salmonella Typhi ‘Vi’ Antigen Specific Bacteriophages
Collection of Water Samples and Removal of Contaminants
A total of five different randomly selected water samples were screened for the presence of bacteriophages against S. Typhi. To isolate bacteriophages, water samples were collected from five different sources- Bhagwa Nala (sewage), Assi drainage, Ravidas park drainage, Durgakund pond and river Ganga water from Assi ghat.
To remove contaminants, each water sample was centrifuged at 10,000 rpm for 10 minutes at 4°C. The supernatant was treated with 1% chloroform solution, and then recentrifuged. The resultant supernatant was collected in fresh eppendorf for further processing. The S. Typhi strain for the experiment was identified by different biochemical tests [12]. The host bacterium was also tested for ‘Vi’ antigen identification by the slide agglutination test [13].
Plaque Assay
Salmonella Typhi ‘Vi’ antigen specific bacteriophages were isolated from the water sources using the “double agar overlay method” [14]. The titre of phages was determined through plaque assay by this technique. Serial dilution was done for each phage suspension obtained after sensitisation.
In sterile test tubes 100 μL of above supernatant was added to 900 μL of Tris-HCl Magnesium Sulphate Gelatine (TMG) buffer and was subsequently serially diluted tenfold from 10-2 to 10-10 and 10 μL of S. Typhi culture in log phase was added in each test tube. All the test tubes were mixed and incubated at 37°C for 20 min in water bath shaker to allow the attachment of phage to host bacteria more efficiently than in agar. Then 5 mL of molten soft agar (cooled at 50°C) was added and mixed in each test tube and poured quickly on top of the solidified agar plate. Number of plaques was observed after incubating the plates overnight at 37°C.
Bacteriophage Purification: PEG Precipitation
Phages were purified by method reported by Yamamoto KR et al., in the year 1970 with some modifications [15]. Polyethylene Glycol (PEG) precipitation is based on the principle of density gradient centrifugations. The supernatants, collected in previous step, were mixed with PEG-6,000/2.5 M NaCl solution, so that, the final concentration of PEG solution (25%) was added to phage supernatant (7.5 mL PEG+30 mL supernatant). It was incubated at 4°C overnight.
After overnight incubation solution was centrifuged at 15,000 rpm for 20 min at 4°C and milky pellet was collected. Phage was recentrifuged 2 to 3 times to remove all of PEG solution and suspended in STE (1,000 μL). DNase treatment was done. Three μL of DNase (10 mg/mL) (Bangalore Genei, Bangalore, India) was added and incubated at 37°C for 30 min. Then 3 μL of Proteinase K (20 mg/mL) (Bangalore Genei, Bangalore, India) was added and incubated at 37°C for 1 hour to inactivate DNase enzyme. This purified suspension was stored at 4°C for further analysis.
Phage DNA Extraction
DNA was isolated by using standard protocol for phage DNA isolation with some modification [16]. Purified milky pellets of phage particles were treated with 0.5% SDS (wt/v), 20 mM EDTA and 2 μg/mL proteinase K at 56°C for 1 hour followed by phenol-chloroform DNA extraction and NaAc-ethanol precipitation method. The RNAse (0.1 mg/mL) treatment was done for 1 hr at room temperature to remove residual RNA. Phage DNA was stored at -20°C in Tris buffer. Phage DNA was eluted using Silica Kit for DNA isolation (Hi-Media, Mumbai, India) according to the manufacturer’s instructions.
Spectrophotometric Analysis of DNA
The most common technique to determine DNA yield and purity is measurement of absorbance. Purity check-up of DNA sample was done by “NanoDrop” spectrophotometer [17].
Agarose Gel Electrophoresis of Phage DNA
Agarose gel electrophoresis was performed by using the standard protocol for phage DNA [16].
Restriction Analysis
Restriction enzyme analysis of isolated phage DNA was carried out. We used three restriction endonuclease enzymes- EcoR1, Alu1 and BamH1 to digest both the samples [Table/Fig-1] [18]. Restriction enzymes were added to the purified phage DNA in the reaction volume of 20 μL and the contents were mixed by gentle flicking. Then the digestion mixtures were kept overnight at 37°C.
Constituents for restriction analysis.
| Tube | Sample number | Buffer | DNA Conc. (ng/μL) | DNA | EcoR1 | Alu1 | BamH1 | DDW+BSA | Total volume |
|---|
| 1 | 1 | 2 μL | 264.3 | 4 μL | 1 μL | | | 13 μL | 20 μL |
| 2 | 1 | 2 μL | 264.3 | 4 μL | | 1 μL | | 13 μL | 20 μL |
| 3 | 1 | 2 μL | 264.3 | 4 μL | | | 1 μL | 13 μL | 20 μL |
| 4 | 2 | 2 μL | 316.9 | 2.5 μL | 1 μL | | | 14.5 μL | 20 μL |
| 5 | 2 | 2 μL | 316.9 | 2.5 μL | | 1 μL | | 14.5 μL | 20 μL |
| 6 | 2 | 2 μL | 316.9 | 2.5 μL | | | 1 μL | 14.5 μL | 20 μL |
DDW: Double distilled water
Next day the digested products were mixed with 6x loading buffer and loaded on 1% agarose gel prepared in 1X TAE buffer containing ethidium bromide (Hi-Media) at 100V for 2-3 hr. DNA ladder (1 kb) was used as a molecular weight marker to calculate the size of phage DNA fragments. Gel image was taken using gel document system.
Results
The S. Typhi strain for the experiment was identified and confirmed by different biochemical tests. The host bacterium also tested positive for ‘Vi’ antigen identification by the slide agglutination test. Among the five different water samples, we were able to isolate only two bacteriophages with two different plaque morphologies in purified form- small (2 mm) and large (5 mm), that too in the same water sample. Both the plaques were circular in shape. The bacteriophages were produced in bulk and harvested.
PEG Precipitation of Phages
PEG has been used for concentrating and isolating viruses from a variety of environmental samples. It is based on the principle of density gradient centrifugation. In these experiment, phages were purified by method reported by Yamamoto KR et al., in the year 1970 with some modifications [15].
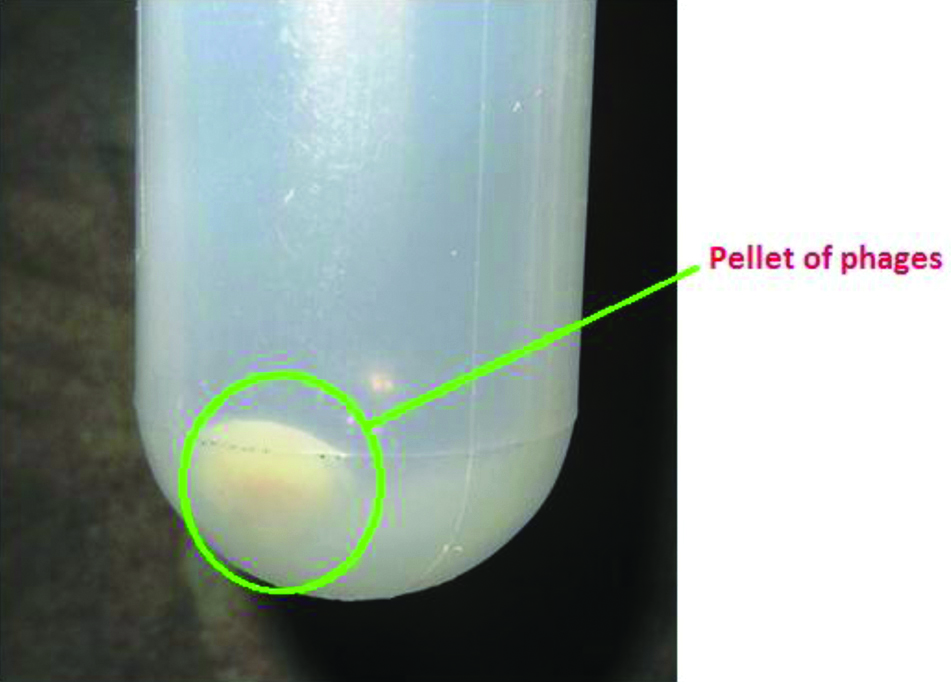
After overnight incubation with PEG-6,000/2.5 M NaCl solution at 4°C, phages were concentrated in the bottom of Oakridge tubes [Table/Fig-2]. On centrifugation at 15,000 rpm for 20 min at 4°C, phages were seen as white milky pellets at the bottom of the tube.
White milky pellet of phage at the bottom of Oakridge tube after PEG precipitation.
Spectrophotometric Analysis of Phage DNA
After DNA extraction, DNA yield and purity check-up of sample was done by “NanoDrop” spectrophotometer. Results obtained are shown in [Table/Fig-3].
“NanoDrop” spectrophotometry readings.
| Sample | DNA Conc. (ng/μL) | Absorbance 260 nm/280 nm | Absorbance 260/230 |
|---|
| 1 | 264.3 | 1.65 | 2.11 |
| 2 | 316.9 | 1.77 | 1.99 |
Results of “NanoDrop” spectrophotometer were suggestive of good quality DNA with minimal protein contamination which could be used in various techniques for molecular characterisation.
Agarose Gel Electrophoresis of DNA Extracted
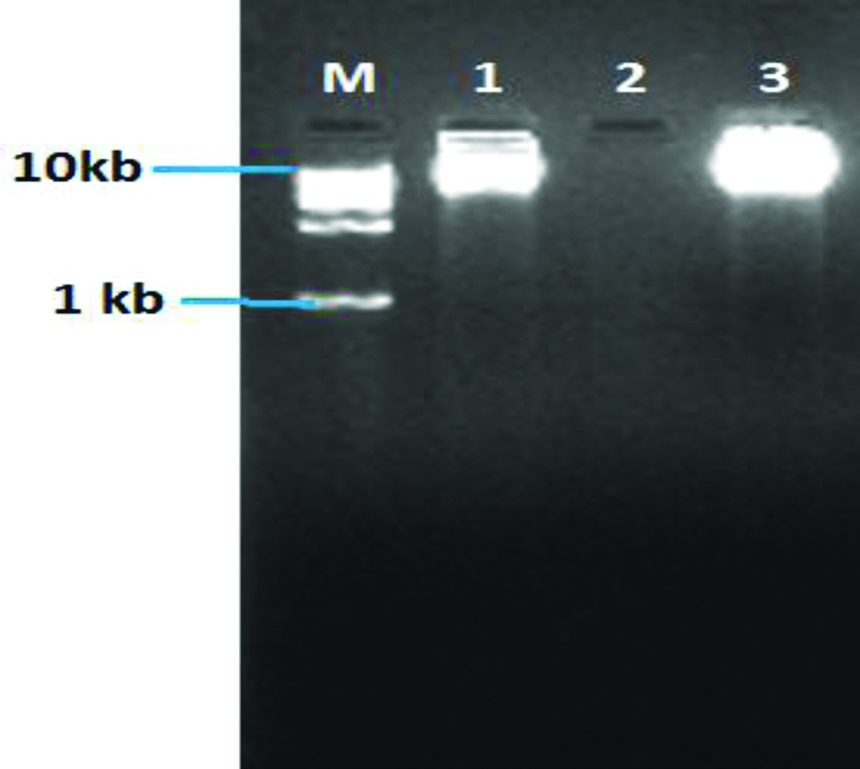
Single thick band confirmed the presence of pure phage DNA [Table/Fig-4]. It was observed that both the phage DNA were approximately more than 10 kb in size.
Gel electrophoresis image of pure genomic DNA of phages.
Lane M- Molecular ladder (1 kb); Lane 1 and 3- Phage genomic DNA; Lane 2- Negative control
Restriction Analysis
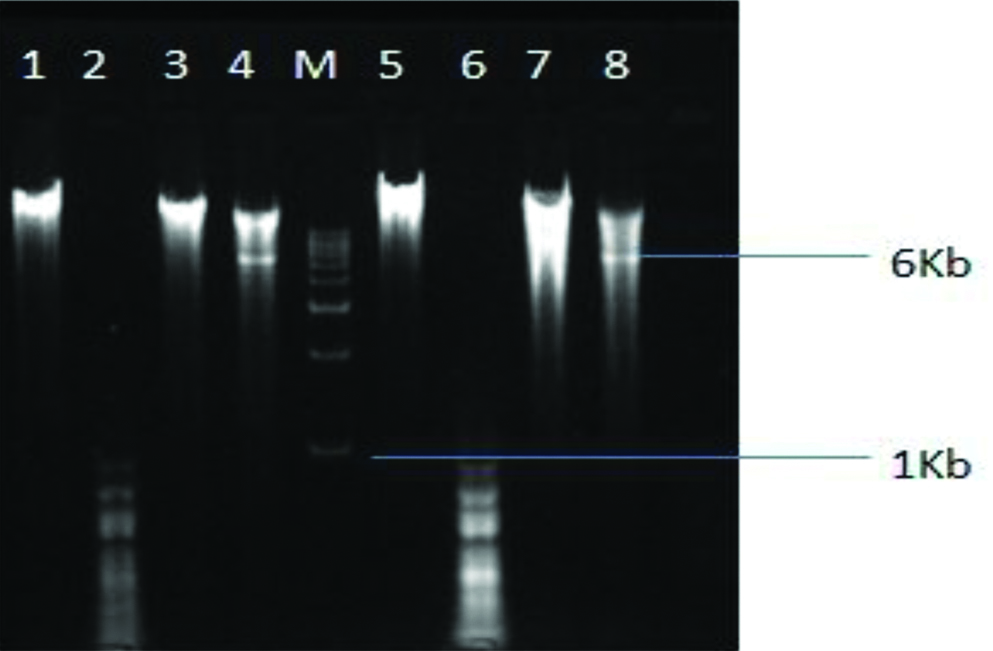
On gel imaging, it was observed that 2 out of 3 restriction enzymes were able to cut both the DNA samples [Table/Fig-5]. Alu1 being a tetracutter was able to cut both the DNA samples into small fragments of less than 1 kb in size while EcoR1 being a hexacutter was able to cut both the DNA samples into fragment of 6 kb in size. BamH1 was not able to cut either of the DNA.
Gel electrophoresis image showing restriction patterns of two phage DNA samples by 3 different restriction enzymes.
Lane M- Molecular ladder (1 kb); lane 1 and 5- pure genomic DNA of phages; lanes 2,3,4, and lanes 6,7,8- products of restriction digestion with Alu1, BamH1 and EcoR1 respectively
Discussion
Since the ancient times, water of the river Ganges has been known for its purity. The reason for this purity has been attributed to the presence of bacteriophages [19]. Bacteriophage for a given bacterium can be isolated wherever that bacterium grows, such as in faeces, sewage, soil, hot springs and oceans, but the good reservoir is still under identification. Phages are generally specific for their host bacterium. However, some phages might act on more than one bacterium.
The applications of phages range from the diagnosis of the disease, through phage typing, and its prevention (phage vaccine), to the treatment (phage therapy). There is hope that phages could be useful to humans in many ways. The multi-drug and pan-drug resistant bacterial infections could be treated with a cocktail of phages using their lytic property. Phages could also be used to deliver a protective antigen and thus could aid in developing vaccines.
In this study, we chose S. Typhi as the host bacterium since typhoid is an endemic disease in this part of the country (North India). Two major problems which are posing challenge in the treatment of this disease are increased drug resistance and chronic carrier state [20]. Various modes of alternative therapy are being tried to deal with them. One of them is the use of bacteriophages against S. Typhi.
‘Vi’ antigen of S. Typhi is an important virulence factor responsible for pathogenicity of disease, development of chronic carrier state and other complications of the disease [21]. The attenuation of the ‘Vi’ antigen could lead to modifying the disease course apparently leading to decreased intensity of disease as well as chronicity [22]. The loss of ‘Vi’ antigen would lead to decreased virulence of the bacterium ultimately leading to decreased chronic carrier status and thus preventing the further dissemination of the organism in the normal population [23].
In the study, five samples of water from three types of sources- pond, sewage, and river including water from river Ganga were taken. When we screened these five water samples for bacteriophages specific to ‘Vi’ positive strain of Salmonella Typhi, then we found presence of bacteriophages in only one source- the Ganga water from Assi ghat. There were two different types of plaque morphologies- large and small, from the same water source, thereby implying that they might be two different types of phages.
On qualitative and quantitative analysis of extracted phage DNA by spectrophotometer, it was found that the quality of the DNA as denoted by its ratio of absorbance at 260 nm/280 nm was 1.65 and 1.77 which is close to the normal ratio (1.8) indicating minimal protein contamination [24]. The DNA concentration was also sufficiently high, making it suitable for various molecular techniques.
On performing agarose gel electrophoresis, both the DNA were found to be more than 10 kb in size and produced single thick band without any smearing, showing that the DNA was pure with minimal degradation and no RNA contamination. The small size of the genomic DNA is suggestive of its viral origin [25].
Molecular characterisation was done by restriction analysis with three restriction enzymes- Alu1, BamH1 and EcoR1. The restriction patterns obtained were similar for both the phages. Alu1 and EcoR1 enzymes restricted both the phages in similar pattern.
Alu1 being a tetracutter was able to restrict both the DNA samples at many sites and gave multiple bands of less than 1 kb in size while EcoR1 being a hexacutter was able to restrict both the DNA samples at a single site and gave a single band of 6 kb size. Another hexacutter BamH1 was not able to restrict either of the DNA. It appears that both the phages were similar genotypically but had different phenotypic expression.
Limitation
The possibility of distinctness of each of the phages cannot be ruled out by doing the restriction analysis alone. The phages need to be further characterised by DNA fingerprinting, protein profiling and whole genome sequencing, however the study was a part of MD thesis and due to financial and time constraint further analysis could not be done. The phages isolated in the present study may also be tested for their therapeutic potential in animal model studies. Transmission Electron Microscopy also needs to be done to better understand the morphological characteristics of the phages.
Conclusion
In the present study, we isolated two bacteriophages targeted against ‘Vi’ positive S. Typhi which were specific only for the host bacterium upon sensitivity analysis. On restriction analysis, both the phages were found to be of the similar pattern, suggesting that they were genetically similar, although they had different plaque morphologies. The use of phages must be shifted from the laboratory to patient care. The genomic characterisation is a pre-requisite and the ‘gold standard’ for any therapeutic use of phages. The present study may be helpful in genomic characterisation of Salmonella Typhi ‘Vi’ specific bacteriophages for their future therapeutic use.
Declaration: Some part of the article has been presented as poster presentation in the ACBICON 2014.