Effect of Fluoxetine on Kidney of Albino Rats- A Histological Study
Alka Aggarwal1, SL Jethani2, RK Rohatgi3, Juhi Kalra4
1 Associate Professor, Department of Anatomy, HIMS, SRHU, Swami Rama Nagar, Jolly Grant, Dehradun, Uttarakhand, India.
2 Professor and Head, Department of Anatomy, HIMS, SRHU, Jolly Grant, Dehradun. Uttarakhand.
3 Professor, Department of Anatomy, HIMS, SRHU, Jolly Grant, Dehradun. Uttarakhand.
4 Professor and Head of Pharmacology, HIMS, SRHU, Jolly Grant, Dehradun, Uttarakhand.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Alka Aggarwal, Associate Professor, Department of Anatomy, HIMS, SRHU, Swami Rama Nagar, Jolly Grant, Dehradun, Uttarakhand, India.
E-mail: alkadr2011@rediffmail.com
Introduction
Fluoxetine and other Selective Serotonin Reuptake Inhibitors (SSRIs) are reported to produce hyponatremia especially in elderly patients.
Aim
To evaluate histological changes in kidney tissue of albino rats by different doses of fluoxetine for different duration and changes in the mean diameter of Proximal Convoluted Tubules (PCT) and Distal Convoluted Tubules (DCT) in control and experimental group was observed.
Materials and Methods
An Experimental study was conducted in which total of 72 albino rats (36 male and 36 female) received single different doses- Mild (10 mg/Kg/day), moderate (20 mg/kg/day) and high (40 mg/kg/day) of intraperitoneal injection of fluoxetine hydrochloride daily for 14 days (2 weeks), 28 days (4 weeks) and 84 days (12 weeks). Changes in kidney tissue histology were investigated and changes in the mean diameter of PCT and Distal convoluted tubules were also observed.
Results
Mild and moderate doses of fluoxetine hydrochloride produce changes in the kidney histology in the form of appearance of podocytes bridge, degenerated/shrunken and swollen glomerulus, dilated tubules when given for long duration. High doses of fluoxetine change kidney histology within few days in the form of hypercellular shrunken glomerulus with apparently increased urinary/capsular space, appearance of podocytes bridge, dilated and degenerated tubules with presence of cast. Mean diameter of PCT was found to be increased in experimental rats in comparison to control rats (p<0.01) but there was no statistical significant change in diameter of DCT.
Conclusion
The findings showed that fluoxetine produce changes in kidney histology both at the level of glomerulus and tubules.
Hyponatremia, Podocyte bridge, Selective serotonin reuptake inhibitors, Syndrome of inappropriate secretion of anti diuretic hormone
Introduction
SSRIs are most widely prescribed anti-depressant drugs for psychiatric diseases. Fluoxetine is a prototype drug of SSRIs group of antidepressants. SSRIs inhibit the presynaptic serotonin or 5-Hydroxytryptamine (5-HT) Reuptake Transport (SERT) system of serotonergic neurons [1,2]. Normally in serotonergic neurons after the release of serotonin, a portion of it is recycled by reuptake into presynaptic serotonergic neurons [3]. The inhibition of SERT leads to an acute increase in serotonin concentrations at the synaptic cleft. The accumulated serotonin binds to the post-synaptic serotonin receptors and produces its effects. Because SSRIs are highly specific for 5-HT receptors and have less effect on other neurotransmitters and channels, these have better safety profile and fewer side effects in comparison to other antidepressants [1,2].
In a review article, Jacob S and Spinler SA included various case reports, observational studies, case-control studies and clinical trials and have documented SSRIs associated hyponatremia (low sodium level) and the incidence varies from 0.5% to 32% [4]. The risk increases in low body weight, elderly, female, associated use of diuretic drugs, and lower baseline concentration of serum sodium [4]. In old patient slow serum sodium developed within 1st and 3rd week after starting the therapy but in the younger patient’s hyponatremia developed after 3rd week of therapy [5]. In elderly people normal level of sodium (normonatremia) achieved within 2 weeks after discontinuation of drug [4].
In mild to moderate hyponatremia, serum sodium concentration remain between 120 to 135 mEq/L and symptoms are headache, blurred vision, polydypsia, weakness, muscle pain, cramps, tremor, impaired gait, feeling of listlessness, tiredness, nausea, vomiting, reduced appetite, confusion progressing to disorientation, agitation and psychosis. Most of these symptoms are non-specific and similar to signs and symptoms of depression [6]. Usually, these similarities in signs and symptoms may lead to under diagnosis and mismanagement usually. In severe hyponatremia serum sodium concentration falls <120 mEq/L and lead to seizures, coma, respiratory arrest. Neurological effects of severe hyponatremia are potentially dangerous and emerge as medical emergency [7,8].
Different previous clinical studies reported cases of hyponatremia in old ladies who developed weakness, nausea, confusion, vomiting progressed to unresponsiveness, generalised seizures on 9th day after they have started 20 mg/day fluoxetine hydrochloride for depression. These cases were diagnosed as a case of fluoxetine induced Syndrome of Inappropriate Secretion of Anti-Diuretic Hormone (SIADH). Hyponatremia and mental status gradually became normal on discontinuation of therapy [9-11]. The rapidly progressive hyponatremia leads to serious neurological dysfunction and accumulation of fluid inside the body’s cells especially in elderly people because their body becomes unable to maintain fluid levels [11].
Fluoxetine is mainly excreted in urine. Kidney plays a central role in maintaining homeostasis by conserving water and electrolytes and disposing metabolic waste [12,13]. There were no previous histological studies which investigate whether fluoxetine effect the kidney’s histology during its excretion. This study was done to see the changes in kidney histology by different doses for different duration of Intraperitoneal (I/P) injection of fluoxetine in albino rats. The present study is the part of previously published study [14].
Materials and Methods
An Experimental study was carried out in the Department of Anatomy of Himalayan Institute of Medical Sciences (HIMS), Dehradun (Uttarakhand) for a period of twelve months (May 2009- April 2010). A total of 72 albino rats (36 males and 36 females) of Rattus norwegicus strain weighing approximately 120-160 grams were used as the experimental animals. All the experimental animals were taken from the Central Animal House of the institute. Study was started after obtaining the approval of Institutional Animal Ethical Committee (IAEC-(Registration No.589/02/a/CPCSEA).
Disease and disability free rats were taken for this study. Throughout the experiment they were allowed free access to standard balanced diet and water ad libitum from the Central Animal House. In the cages, they were kept with a 12 hour: 12 hour light-dark cycle. The drug Fluoxetine hydrochloride (Cap. Flunil-20 mg, INTAS Pharmaceuticals), 20 mg was dissolved in 2 mL of normal saline to make 10 mg/mL drug concentration. The drug was injected I/P, according to weight of the rat, once in a day in 3 phases-Phase-1 (2 weeks), Phase-2 (4 weeks) and Phase-3 (12 weeks) duration. Each phase consisted of 24 animals. These 24 animals were further randomly subdivided into 4 Groups. Each group consists of 6 albino rats (3 males and 3 females). Group 1 (Control) received I/P injection of vehicle (Normal saline). Group 2, group 3 and group 4 rats were injected with 10 mg/kg/day, 20 mg/kg/day and 40 mg/kg/day of body weight/day of fluoxetine respectively. All rats were weighed on alternate day for drug dose calculation and growth monitoring [14].
The control group and experimental group rats were sacrificed at the end of 2nd week, 4th week and 12th week after giving ether anaesthesia. Dissection was done and kidney tissues were procured and fixed in 10% formalin. A 3-5 mm thick tissue slices were taken and processed. They were sectioned (4-5 μ thickness) and stained with Harris Haematoxylin and Eosin (H&E) for histological analysis under light microscope. Microscopic examination was done under X200 magnification.
Statistical Analysis
The data collected was statistically analysed with the help of Microsoft Excel version 2007 (Mean, Standard Deviation (SD) and Student’s t-test was used.
Results
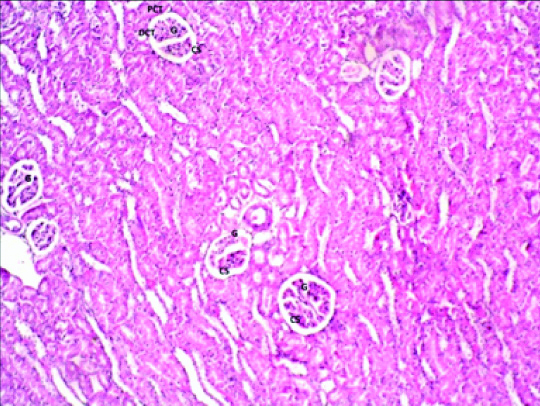
On histological examination of group 1 (control), there was presence of spherical renal corpuscle. Visceral and parietal layer of Bowman’s capsule are made up of podocytes lining the glomerular capillaries and simple squamous epithelium respectively. In between the two layers, there are space known as Glomerular (urinary) Space (GS). Lining epithelium of PCT, DCT, thin limb (ascending and descending) and thick limb of loop of henle was simple cuboidal epithelium with microvilli, simple cuboidal epithelium without microvilli, simple squamous epithelium and simple cuboidal epithelium respectively. Due to presence of microvilli, lumen appears narrow in PCT in comparison to DCT [Table/Fig-1].
Photomicrograph of the Kidney of Group1 (control) albino rat showing glomerulus (G), Glomerular space (GS), Proximal convoluted tubule (PCT), Distal convoluted tubule (DCT) (H&E stain X 200).
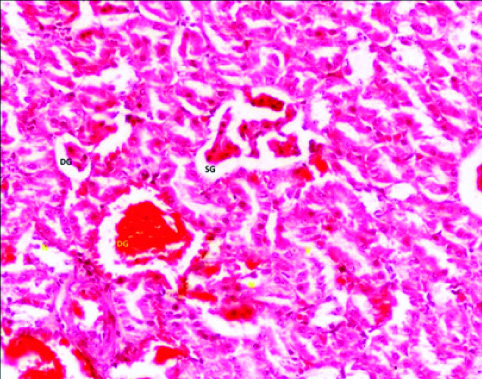
In group 2, (10 mg/kg/day)-phase 3 (12 weeks) albino rats; there was change in kidney histology in the form of dilation of tubule. Glomerulus appear scattered (swollen) and degenerated at some places, Glomerular Space (GS) were reduced. At some places hyperemia was also found [Table/Fig-2].
Photomicrograph of the Kidney of Group 2 (10 mg/kg/day) Phase-3 (12 weeks) of Experimental albino rats showing scattered glomerulus (SG), Degenerated glomerulus (DG). (H&E stain X 200).
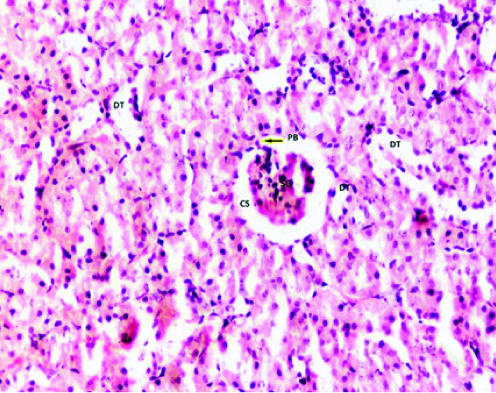
In group 3 (20 mg/kg/day), there was not much change in kidney’s histiology in phase 1 (2 week) but in phase 2 (4 week) albino rats there was dilation of tubules with presence of podocytes in between the glomerular capillary tuft and parietal layer of bowmans capsule-Podocyte Bridge (PB). The glomerular capillary tuft appears hypercellular. In phase 3 (12 weeks), there was shrunken glomerulus with its attachment to the parietal layer of bowman’s capsule [Table/Fig-3].
Photomicrograph of the Kidney of Group 3 (20 mg/kg/day) Phase-3 (12 weeks) of Experimental albino rats showing Dialated tubule (DT), Shrunken glomerulus (SG), with capsular space (CS), Podocyte bridge (arrow). (H&E stain X200).
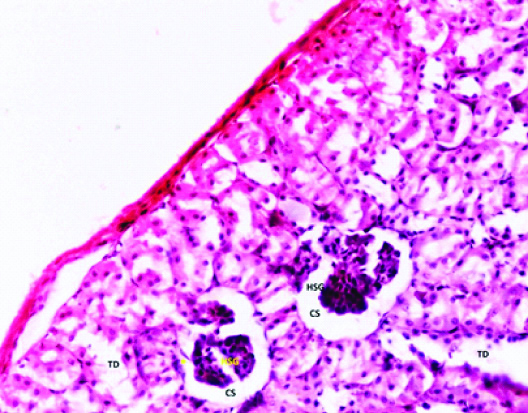
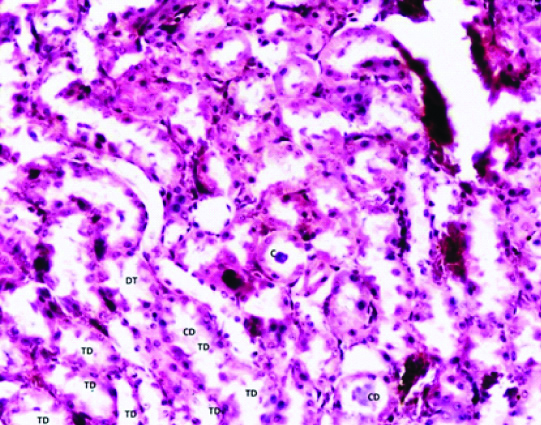
In group 4 (Phase 1) albino rats; the tubules were found dilated with disturbed cellular orientation. There was presence of cellular debris-cast (C) in the lumen of tubules. The glomerular tuft appears shrunken and attached to the parietal layer of bowman’s capsule with wide glomerular space [Table/Fig-4,5].
Photomicrograph of the Kidney of Group 4 (40 mg/kg/day) Phase-1 (2 weeks) of Experimental albino rats showing Hypercellular shrunken glomerulus (HSG), Capsular space (CS), Podocyte bridge (Yellow star), Tubular degeneration (TD). (H&E stain X200).
Photomicrograph of the Kidney of Group 4 (40 mg/kg/day) Phase-1 (2 weeks) of Experimental albino rats showing cast (C) in the lumen of Collecting duct (CD), Dialated tubules (DT), Tubular degeneration (TD). (H&E stain X200).
The diameter of PCT was increased in Group 2 albino rats in all phases but in Group 3 PCT diameter was increased at the end of 2 weeks but the diameter was decreased in Group 3 at the end of 4 weeks and 12 weeks. The survived rats of Group 4 of 2 weeks also showed the increase in PCT diameter [Table/Fig-6].
Comparative changes in the mean diameter of Proximal Convoluted tubules in Control and Experimental Groups.
| Groups | 2 weeks (Mean±SD) | 4 weeks (Mean±SD) | 12 weeks (Mean±SD) |
|---|
| Group 1 (Control) | 10±1.154 | 11.1±0.875 | 12.9±2.02 |
| Group 2 (10 mg/kg/day) | 12.1±3.665 p=0.073 | 14.8±3.82 p=0.009 | 15.4±2.8 p=0.004 |
| Group 3 (20 mg/kg/day) | 16.8±1.23 p=0.0000004 | 12.7±1.82 p=0.024 | 13.3±3.02 p=0.363 |
| Group 4 (40 mg/kg/day) | 13.8±2.34 p=0.002 | 0 | 0 |
(All numerical data were subjected to student’s t-test)
Changes in the diameter of PCT were observed in the present study. The diameter of DCT did not change significantly. The diameter of DCT in Group 2 experimental albino rats was found decreased slightly in comparison to same phase control. In Group 3 (20 mg/kg/day) albino rats a slight decrease in DCT diameter was observed at the end of 2, 4 and 12 weeks respectively in comparison to control of same group. The rats which survived in Group 4 (40 mg/kg/day) did not show much change [Table/Fig-7].
Changes in the mean diameter of Distal convoluted tubules in Control and Experimental Groups.
| Groups | 2 weeks (Mean±SD) | 4 weeks (Mean±SD) | 12 weeks (Mean±SD) |
|---|
| Group 1 (Control) | 11±2.21 | 11.1±2.33 | 12.4±2.41 |
| Group 2 (10 mg/kg/day) | 9.8±1.54p=0.093 | 10.3±1.05p=0.163 | 11.6±1.2p=0.01 |
| Group 3 (20 mg/kg/day) | 10.8±0.92p=0.404 | 10.8±0.918p=0.363 | 10.6±1.71p=0.079 |
| Group 4 (40 mg/kg/day) | 11.3±1.49p=0.331 | 0 | 0 |
(All numerical data were subjected to student’s t-test)
In the present study, most of the rats of Group 4 died within 2 weeks. These rats developed muscle twitching, sluggish movements during initial days of drug administration and later on they died.
Discussion
Nephron is the structural and functional unit of paired kidney. The endothelium of glomerular capillary, Glomerular Basement Membrane (GBM) and the podocytes of visceral layer of Bowman’s capsule together form the filteration apparatus. Foot processes (pedicle) of one podocyte interdigitate with foot process of the neighboring podocytes and the spaces between interdigitating foot processes form filteration slit [13].
Authors reported morphologic alterations in endocapillary compartment without any appeared effect on podocytes after giving intravenous injection of rabbit’s anti-glomerular basement membrane serum to mice on 3rd day. On 6th day, there were widespread alterations of podocytes in the form of podocytes adhered to glomerular basement membrane form bridges between the glomerular tuft and parietal layer of Bowman’s capsule-Podocyte Bridge (PB) with crescents formation in few glomeruli. On 10th day, crescents were found in 40% of glomeruli. They interpreted this that after a lesion of the parietal epithelium, the glomerular podocytes spread on the parietal basement membrane. The PB initiates crescent formation by proliferation of Parietal epithelial cells (PECs) [15]. In the present study, PB were found in the Group-2 (10 mg/kg/day) Phase-3 (12 week), Group-3 (20 mg/kg/day) Phase-3, and Group 4 (40 mg/kg /day) phase -1 albino rats [Table/Fig-2,3 and 4].
It was reported that in degenerative and inflammatory diseases, nephron loss starts from glomerulus in the form of loss of the separation between tuft and Bowman’s capsule by formation of PB. Tubular injury remains secondary to glomerular lesions. PB initiates the formation of crescents. Podocytes play an important and central role. Endocapillary compartment injuries usually repaired by scarring [16]. In present study, it was also found that glomerulus was adhered to the parietal layer. But we could not find crescent formation although there were tubular changes in the form of dilatation of tubules.
A histopathological study reported changes in kidney in SIADH in the form of dilatation of renal tubules associated with degenerative changes in the malpighian corpuscles in the form of contracted glomeruli and narrowed out Bowman’s capsule in the renal cortex. They also found that inflammatory cells infilteration in the kidney tissue, basophilic reaction in tubules, and mineralization of kidney tissue which lead to increased weight of kidneys [17]. In the present study, inflammatory infilterate and mineralization of kidney tissue were not found. Dilatation of tubules with contracted glomeruli were seen. In some tubules, there were cells within the lumen in group 4 (40 mg/kg/day phase-1 albino rats [Table/Fig-4,5].
Limitation
The present study limits only to histological and few morphometric findings.
Conclusion
The present study showed changes in glomerulus and also in tubules. It was reported and seen that glomerular and tubular changes effect the normal filtration and reabsorption process which may lead to filtration of RBCs, proteins, electrolytes and casts in urine. The point of further investigation is whether changes in kidney’s histology may be primary action of SSRIs or it may be secondary to SIDAH produced by SSRIs. The elderly patients on the moderate or high doses of SSRIs should be monitored and investigated for levels of electrolytes in blood, RBCs, protein and casts in urine.
(All numerical data were subjected to student’s t-test)
(All numerical data were subjected to student’s t-test)
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? NA
For any images presented appropriate consent has been obtained from the subjects. NA
PLAGIARISM CHECKING METHODS: [Jain H et al.]
Plagiarism X-checker: Jul 11, 2019
Manual Googling: Sep 10, 2019
iThenticate Software: Sep 25, 2019 (15%)
[1]. Trevor AJ, Katjung BG, Krudering-Hall M, Masters SB, Chapter 30. AntidepressantsIn: Katzung & Trevor’s Pharmacology: Examination and Board Review10th edAccess Pharmacy. McGraw-Hill Medicalhttps://accesspharmacy.mhmedical.com/… [Google Scholar]
[2]. Tripathi KD, Drugs acting on Central Nervous system. Chapter 33. Drugs used in mental illness: antidepressant and antianxiety drugsIn: Essentials of Medical Pharmacology 2013 7th edNew Delhi. IndiaJaypee Brothers Medical Publishers (P) Ltd:454-68.10.5005/jp/books/12256_35 [Google Scholar] [CrossRef]
[3]. Ross MH, Pawlina W, Chapter 12. Nerve TissueIn: Histology: A text and atlas with correlated cell and molecular biology 2016 7th edPhiladelphiaWolterskluwer:356-393. [Google Scholar]
[4]. Jacob S, Spinler SA, Hyponatremia associated with selective serotonin-reuptake inhibitors in older adultsAnn Pharmacother 2006 40(9):1618-22.10.1345/aph.1G29316896026 [Google Scholar] [CrossRef] [PubMed]
[5]. Fereshteh S, Ali G, Mahmood A, Khalil T, Serum sodium changes in fluoxetine users at different age groupsIran J Psychiatry 2010 5(3):113-16. [Google Scholar]
[6]. Reynolds RE, Padfield PA, Seckle JR, Disorders of sodium balanceBMJ 2006 332:702-05.10.1136/bmj.332.7543.70216565125 [Google Scholar] [CrossRef] [PubMed]
[7]. Miranda M, Armijo G, Miranda P, Severe hyponatremia during treatment with fluoxetineRev Med Chil 1999 127:337-40. [Google Scholar]
[8]. Romerio SC, Radanowicz V, Schlienger RG, SIADH with epileptic seizures and coma in fluoxetine therapyPraxis 2000 89:404-10. [Google Scholar]
[9]. Twardowschy CA, Bertolucci CB, GraciaCde M, Brandao MA, Severe hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) associated with fluoxetine: case reportArq Neuropsiquiatr 2006 64(1):142-45.https://www.ncbi.nlm.nih.gov/.../166225...10.1590/S0004-282X200600010003116622573 [Google Scholar] [CrossRef] [PubMed]
[10]. Girault C, Richard JC, Chevron V, Goulle JP, Droy JM, Bonmarchand G, Syndrome of inappropriate secretion of antidiuretic hormone in two elderly women with elevated serum fluoxetineJ Toxicol Clin Toxicol 1997 35(1):93-95.10.3109/155636597090011729022659 [Google Scholar] [CrossRef] [PubMed]
[11]. Vivek CK, Prashant PJ, Syndrome of Inappropriate ADH Secretion (SIADH) associated with citalopram useIndian J Psychiatry 2005 47(2):119-20.10.4103/0019-5545.5596020711296 [Google Scholar] [CrossRef] [PubMed]
[12]. Sangkuhl K, Fluoxetine Pathway, Pharmacokinetics overview. In pharmacogenomic knowledge for personalized medicineClinical pharmacology and therapeutics 2012 https://www.pharmgkb.org> pathway [Google Scholar]
[13]. Ross MH, Pawlina W, Chapter 20. Urinary systemIn: Histology: a text and atlas with correlated cell and molecular biology 2016 7th edPhiladelphiaWolterskluwer:698-741. [Google Scholar]
[14]. Aggarwal A, Jethani SL, Rohatgi RK, Kalra J, Selective Serotonin Re-uptake Inhibitors (SSRIs) Induced Weight changes: A Dose and Duration Dependent Study on Albino RatsJCDR March 2016 10(3):AF01-AF03.10.7860/JCDR/2016/16482.737627134853 [Google Scholar] [CrossRef] [PubMed]
[15]. Le Hir M, Keller C, Eschmann V, Hahnel B, Hosser H, Kriz W, Podocyte bridges between the tuft and bowman’s capsule: an early event in experimental crescentic glomerulonephritisJ Am Soc Nephrol 2001 12(10):2060-71. [Google Scholar]
[16]. Kriz W, lehir M, Pathways to nephron loss starting from glomerular diseases- insights from animal modelsKidney International 2005 67(2):404-19.10.1111/j.1523-1755.2005.67097.x15673288 [Google Scholar] [CrossRef] [PubMed]
[17]. Naito A, Hasegawa H, Kurasawa T, Ohtake Y, Matsukawa H, Ezure Y, Histopathological study of kidney abnormalities in an experimental SIADH rat model and its application to the evaluation of the pharmacologic profile of VP-343, a selective vasopressin V2 receptor antagonistBiol Pharm Bull 2001 24(8):897-901.10.1248/bpb.24.89711510481 [Google Scholar] [CrossRef] [PubMed]