Epilepsy remains the major nervous system disorder attacking people from childhood to old age. Approximately, 50 million people worldwide and around 1 crore people in India are epileptic [1,2]. Epilepsy is the pathologic and enduring tendency of the brain to have recurrent seizures [3]. Self-annihilation, fear, stress, depression and accidental death are common among epilepsy patient’s [4].
Seizure cessation and prevention of ADRs thus remains the ultimate goal of epilepsy treatment. Optimum therapeutic benefit requires long term commitment and compliance to AEDs [5]. Despite low cost and wide availability of older AEDs, newer AEDs are the preferred choice of epileptologists [6]. In the past decade much research has been focused on either comparing drugs of monotherapy or comparing older anti-epileptics to the newer ones [7,8]. Fewer studies have focussed on the health related QOL of the patient and the ADRs associated with the prescribed medications [7,8]. Further, these studies did not evaluate all the drugs for effect on seizure control and variables that may act as predictors for seizure control.
Thus, present study was designed to evaluate effectiveness of AEDs, predictors of seizure control, ADRs, medication adherence and QOL in epilepsy patients.
Materials and Methods
An observational cohort pilot study was performed on generalised tonic clonic and focal epilepsy patients for six months (December 2017-May 2018) in the Neurology Department of Owaisi Hospital and Research Centre, Hyderabad, Telangana, India. Institutional Ethical Review Board acceptance (IRB approval number 2017/19/006) was procured. A sample of 100 was selected in which 81 patients met the inclusion criteria who were receiving any of the five AEDs; Levetiracetam, Phenytoin, Sod. Valproate, Oxcarbazepine, and Lacosamide as Monotherapy for atleast six months.
Selection Criteria and Data Collection
Male and female epilepsy patients, aged 10 years or older receiving AEDs and accepting to participate in the study were included. Patients that were pregnant, patients with a reported head injury, drug induced epileptic seizures, patients with progressive neurological diseases, patients with mental disorders, severe psychological problems, strokes, cerebral tumours, and patients who have had recent brain operation were excluded.
A patient profile sheet specially designed [Annexure-1] was used to collect data on therapeutic, socio-demographic and clinical parameters. The medication data collected comprised of generic/brand name, daily dose and safety profile after the administration of the drug.
Measures
Drug monitoring for seizure control: In patients that continued to have seizures, an increased dose was considered up to maximum level as long as no unwanted effects occurred. In patients having adverse effects that are dose-related, a dose reduction was considered. All the patients were followed-up weekly for six months for treatment response and adverse effects.
The ADR profile: The ADR profile includes: [i] type of ADR; [ii] The causality relationship of the ADR with suspected drug according to Naranjo ADR probability scale [9].
Assessment of medication adherence: The Morisky Medication Adherence Scale-4 was used to measure the medication adherence of the patient before and after patient counselling. Patient counselling was done individually by the study investigators. The Morisky Medication adherence scale uses a 5 point scale from 0 to 4 to categorise the patient as non-adherent, moderately adherent and completely adherent [10].
The QOL assessment: The QOLIE-31 questionnaire was used to assess the QOL every four weeks. The QOLIE-31 consists of seven item scales, consisting of seizure anxiety, emotional satisfaction, energetic/restlessness, memory, treatment effects, community based effects, health status and QOL [11]. The questionnaire translations were delivered to patients in English, Hindi, Urdu and Telugu and the answers were documented in patient information sheet.
Statistical Analysis
Descriptive statistical analysis was carried out using MS excel (2016 version) spread sheet to generate graphs, tables, etc., for the study. All statistical analysis was performed using Epi Info software version 7.0 (CDC., Atlanta, Georgia, USA). Continuous data were presented as mean±SD and categorical data was presented as frequencies and percentages. Continuous variables were compared using student’s t-test and analysis of variance (ANOVA). Statistical significance was set at a two sided p-value of < 0.05. Linear regression was used to find the effect of predictor variables on QOLIE t-scores. Logistic regression was used to evaluate the effect of various predictor variables on seizure control. Associations were evaluated by correlation coefficients and odds ratios for linear and logistic regression respectively with 95% confidence intervals.
Results
Baseline Characteristics
A total of 81 patients were medically examined during the study period in the Neurology Department of Owaisi Hospital and Research Centre, Hyderabad and achieved the inclusion criteria within the study duration. Summary of the patient characteristics have been shown in [Table/Fig-1].
Socio-demographic profile of the patients.
| Parameters | Category | N=81; n (%) |
|---|
| Sex | Male | 44 (54.3) |
| Female | 37 (45.6) |
| Age (years) | Mean age±sd | 42.691±19.481 |
| Median | 44 |
| Marital status | Married | 60 (74) |
| Unmarried | 21 (25.9) |
| Employment status | Employed | 26 (32) |
| Unemployed | 55 (67.9) |
| Smoking | Smokers | 19 (23.4) |
| Non-smokers | 62 [76.5] |
| Alcohol | Alcoholic | 9 (11.1) |
| Non-alcoholic | 72 [88.8] |
The majority were males (54%) and the median age was 44 years. Type of seizure was predominantly generalised with 85% of the patients and 15% with focal epilepsy. Most number of patients (18) was recorded in the age group of 50-59 and the least (3) in the age group of 80-90. Majority of males (10) and females (8) were reported in the age group 50-59. The majority of the population was married and unemployed [Table/Fig-1].
Assessment of Medication Adherence
Medication adherence in patients before patient counselling differed significantly after given patient counselling. [Table/Fig-2] shows the difference in the MMAS (Morisky Medication Adherence Score) from the AED before and after three months of patient counselling.
Medication adherence from AED use before and after patient counseling.
| Drug | MMAS (before) | MMAS (after) | p-value (paired t-test) |
|---|
| Levetiracetam | 1.82±0.69 | 3.56±0.49 | p=0.0001 |
| Phenytoin | 1.66±0.74 | 3.50±0.50 | p=0.0001 |
| Sod. Valproate | 1.98±0.45 | 3.71±0.45 | p=0.0001 |
| Oxcarbazepine | 2.33±0.74 | 3.83±0.37 | p=0.002 |
| Lacosamide | 2.11±0.73 | 3.77±0.41 | p=0.0005 |
Before and after MMA scores were compared using Paired t-test
AED Treatment Profile
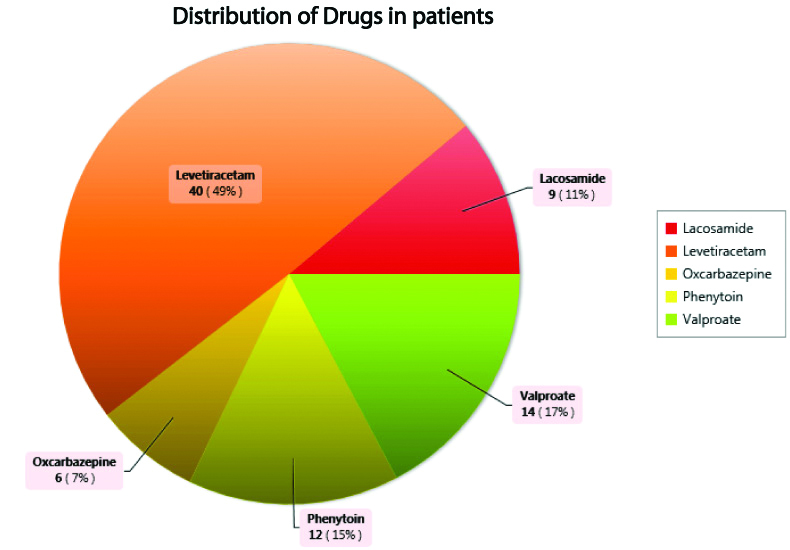
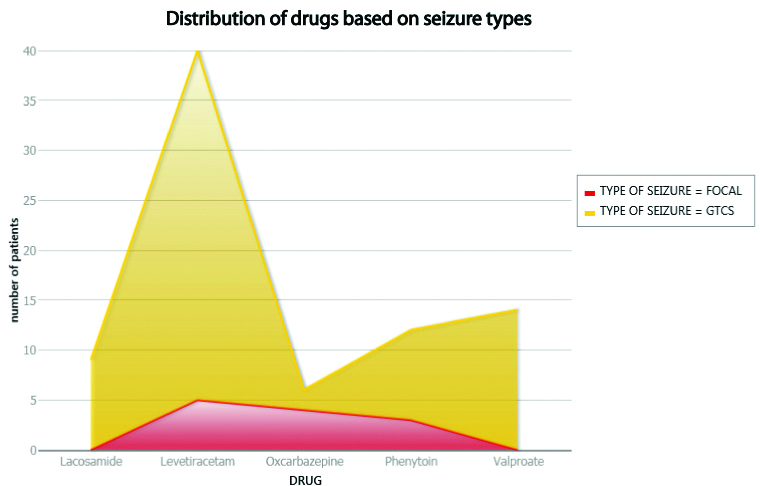
A total of 81 patients received AEDs as monotherapy. The percentage of patients with generalised seizures (69) and focal seizures (12) were found to be 85% and 15%, respectively. The most frequently received AED was Levetiracetam in 49% and least was Oxcarbazepine in 7% of the patients [Table/Fig-3,4].
Distribution of drugs in patients.
Distribution of drugs based on seizure types.
ADR Profile
Sodium valproate was associated with higher number of ADRs [Table/Fig-5]. Majority of ADRs involved the gastrointestinal system followed by the psychiatry and central nervous system. A serious ADR reported with phenytoin was drug induced hypersensitivity reaction. [Table/Fig-6] indicate the frequently reported ADRs from the AEDs and their causality assessment from the Naranjo Assessment Scaling of Probable, Possible and Definite.
Distribution of ADRs based on drugs.
| Adverse effects | AED | Total |
|---|
| LEVIPIL[AD: 500 mg] | ATOIN[AD: 100 mg] | LACOSET[AD: 100 mg] | OLEPTAL[AD: 150 mg] | NAVALIN[AD: 200 mg] |
|---|
| Dizziness | - | 1 | 2 | - | 1 | 4 |
| Headache | 4 | - | - | - | - | 4 |
| Somnolence | 1 | - | - | 1 | - | 2 |
| Irritability | 4 | 1 | 1 | - | - | 6 |
| Aggression | - | - | 1 | - | - | 1 |
| Rash | - | 2 | - | - | - | 2 |
| Hair loss | - | 1 (Focal epilepsy) | - | - | - | 1 |
| Nausea | 1 | - | - | - | 7 | 8 |
| Fatigue | - | - | 2 | - | - | 2 |
| Back pain | - | - | - | 2 (Focal epilepsy) | - | 2 |
| Fever | - | 1 | - | - | - | 1 |
| Weight gain | - | - | - | - | 2 | 2 |
LEVIPIL: Levetiracetam; ATOIN: Phenytoin; LACOSET: Lacosamide; OLEPTAL: Oxcarbazepine; NAVALIN: Sodium Valproate; *AD: Average Dose; Only Back pain and Hair loss were ADRs found in Focal epileptic patients; Rest all the ADRs were seen in generalised epilepsy patients
Frequently reported ADRs.
| Drug | Frequently reported ADR | Naranjo scale |
|---|
| Levetiracetam | Headache | Probable |
| Irritability | Probable |
| Phenytoin | Rash | Definite |
| Hypersensitivity reactions | Probable |
| Sodium Valproate | Weight gain | Possible |
| Nausea | Possible |
| Oxcarbazepine | Somnolence | Possible |
| Back pain | Probable |
| Lacosamide | Fatigue | Probable |
| Dizziness | Possible |
Seizure Control
Seizure Control was 100% with Oxcarbazepine followed by Lacosamide with 89% and Levetiracetam with 80% [Table/Fig-7,8].
Seizure control based on drug.
| Drug | Frequency | Percent |
|---|
| Lacosamide | 8/9 | 89% |
| Levetiracetam | 32/40 | 80% |
| Oxcarbazepine | 6/6 | 100% |
| Phenytoin | 9/12 | 75% |
| Sodium Valproate | 9/14 | 64% |
| Total | 64 | 100.00% |
Seizure control based on gender.
| a) Sex=Female |
|---|
| Seizure control | Frequency | Percent | Cum. percent | Exact 95% LCL | Exact 95% UCL |
|---|
| No | 5 | 13.51% | 13.51% | 4.54% | 28.77% |
| Yes | 32 | 86.49% | 100.00% | 71.23% | 95.46% |
| Total | 37 | 100.00% | 100.00% | | |
| b) Sex=Male |
| Seizure control | Frequency | Percent | Cum. percent | Exact 95% LCL | Exact 95% UCL |
| No | 12 | 27.27% | 27.27% | 14.96% | 42.79% |
| Yes | 32 | 72.73% | 100.00% | 57.21% | 85.04% |
| Total | 44 | 100.00% | 100.00% | | |
QOL Assessment and Seizure Control
From the overall QOL scoring, optimal or high QOLIE score was reported in majority of the patients implying better QOL. Generalised and partial seizures did not differ significantly with respect to QOL score. QOLIE scoring differed significantly between groups of drugs, with high QOL seen in Levetiracetam, while it was least in patients receiving phenytoin [Table/Fig-9]. None of the predictors were able to explain QOL [Table/Fig-10]. In the multivariable model, only age was significantly associated with seizure control [Table/Fig-11].
QOLIE T scores based on drug.
| a) QOLIE T score |
|---|
| QOLIE T score * drug | Obs | Total | Mean | Var | Std Dev | Min | 25% | Median | 75% | Max | Mode |
|---|
| Lacosamide | 9 | 529 | 58.7778 | 19.4444 | 4.4096 | 52 | 55 | 60 | 62 | 65 | 55 |
| Levetiracetam | 40 | 2381 | 59.525 | 65.3327 | 8.0829 | 42 | 54.5 | 61.5 | 66 | 71 | 66 |
| Oxcarbazepine | 6 | 319 | 53.1667 | 54.5667 | 7.3869 | 47 | 47 | 50 | 62 | 63 | 47 |
| Phenytoin | 12 | 615 | 51.25 | 124.5682 | 11.161 | 36 | 38.5 | 55.5 | 61 | 65 | 36 |
| Valproate | 14 | 730 | 52.1429 | 56.1319 | 7.4921 | 40 | 46 | 50 | 60 | 63 | 45 |
| b) ANOVA, a Parametric test for inequality of population means. |
| Variation | SS | df | MS | F statistic | | | | | | | |
| Between | 1075.8447 | 4 | 268.9612 | 4.0267 | | | | | | | |
| Within | 5076.3282 | 76 | 66.7938 | | | | | | | | |
| Total | 6152.1728 | 80 | | | | | | | | | |
| p-value | 0.0051 | | | | | | | | | | |
*drug name
Predictors of QOLIE t score.
| Variable | Coefficient | 95% Confidence | Limits | Std error | F-test | p-value |
|---|
| ADR | -3.878 | -8.102 | 0.346 | 2.12 | 3.3463 | 0.071386 |
| Age | 0.002 | -0.101 | 0.105 | 0.052 | 0.0015 | 0.968732 |
| Alcoholic | -1.511 | -8.494 | 5.472 | 3.505 | 0.1858 | 0.667665 |
| Sex | -2.457 | -7.289 | 2.374 | 2.425 | 1.0269 | 0.31419 |
| Smoker | 1.06 | -4.297 | 6.417 | 2.688 | 0.1554 | 0.694536 |
| Type of seizure | 2.626 | -2.977 | 8.229 | 2.812 | 0.8719 | 0.353467 |
| Constant | 58.711 | 52.845 | 64.577 | 2.944 | 397.7222 | 0 |
Correlation coefficient: r^2=0.10
Predictors of seizure control.
| Term | Odds ratio | 95% | C.I. | Coefficient | S.E. | Z-statistic | p-value |
|---|
| ADR (Yes/No) | 0.3409 | 0.0918 | 1.2657 | -1.0762 | 0.6693 | -1.6079 | 0.1079 |
| Age | 0.9528 | 0.9195 | 0.9873 | -0.0484 | 0.0181 | -2.6655 | 0.0077 |
| Alcoholic (Yes/No) | 0.4346 | 0.0739 | 2.5546 | -0.8334 | 0.9037 | -0.9222 | 0.3564 |
| Sex | 2.4887 | 0.5968 | 10.3779 | 0.9117 | 0.7286 | 1.2515 | 0.2108 |
| Type of seizure (Yes/No) | 1.2718 | 0.1968 | 8.2207 | 0.2404 | 0.9522 | 0.2525 | 0.8006 |
| Constant | * | * | * | 3.8531 | 1.0608 | 3.6321 | 0.0003 |
| Convergence: | Converged | | | | | | |
| Iterations: | 4 | | | | | | |
| Final-2*Log-likelihood: | 66.5851 | | | | | | |
| Cases included: | 81 | | | | | | |
| Test | Statistic | D.F. | p-value | | | | |
| Score | 16.6653 | 5 | 0.0052 | | | | |
| Likelihood ratio | 16.6493 | 5 | 0.0052 | | | | |
Discussion
Epilepsy treatment aims to eliminate seizures, minimise adverse effects and improve QOL of patients. Severe adverse effects and continued seizures are sufficient reasons for discontinuation of an antiepileptic drug. Non-compliance with medication of can be the single most common reason for treatment failure and can be changed by proper patient counselling. The treatment success of an epileptic patient is again dependent on enhancement of QOL and tolerability of antiepileptic drug [7,8]. Thus, inclusion of QOL outcomes in treatment plan along with analysis of seizure frequency and ADRs is therefore the need of the time [10]. To address these objectives, present study compared AEDs in terms of seizure cessation, adverse effects and QOL.
Demographic and Clinical Profile
Older age people were scarce in our study as opposed to advanced nations where increasing occurrence of epilepsy is found in elderly people [12]. The number of men was approximately equal to women which were contrary to that described in other studies [13]. There was a high number of unemployed in our study which was similar to a European study that documented a higher unemployment rate [10]. Majority of the seizures were generalised similar to a study that details the prevalence of primary generalised seizures [14]. Epilepsy studies advocate monotherapy as the first line treatment and polytherapy is only preferred when maximum dose of single antiepileptic drug fails to stop seizures [15,16]. Moreover, as most other studies prefer a monotherapy regimen and comparison to polytherapy will be inconclusive, only patients on monotherapy were included in our study [7,15-17].
AED Treatment Profile
Despite high cost, newer antiepileptics have now replaced older AEDs like Phenytoin and Sodium Valproate. The reduced use of older AEDs describes their lower tolerability and significant harmful profile [18]. These results have been replicated by our study which shows that Levetiracetam, Oxcarbazepine and Lacosamide show better seizure control than Phenytoin and Valproate. The increasing use of Levetiracetam observed in present study may be rationalised on the fact that it is useful in various seizure types and is having good safety profile in all age groups. It’s a common assumption that efficacy of newer and older AEDs is similar but safety profile is better with newer AEDs [10,19,20]. While literature search returned many studies, these are limited to one drug versus placebo as monotherapy or between two AEDs which does not allow us to make direct comparison of all AEDs at one time. Moreover, other studies [Table/Fig-12] show Valproate being better than Levetiracetam, Phenytoin and Oxcarbazepine in many cases as opposed to the present study where levetiracetam was found to be better than valproate [17,21-25].
Characteristics of the included studies [17,21-25].
| Reference | Population/design | Duration | Type of seizure | Outcome |
|---|
| Seizure freedom (%) 2 | Therapetic inefficacy (%)1 |
|---|
| Trinka E et al., [21] | Adults/ unblinded, randomised, superiority trial | 12 months | All types | Valproate: 45.5; levetiracetam: 39.5 | Valproate: 3.4; levetiracetam: 2.6 |
| Zhu F et al., [22] | Adults/retrospective observational study | 7 years | Focal epilepsy | Valproate: 36.90; levetiracetam: 40.10; oxcarbazepine:17.20 | Not specified |
| Chung S et al., [17] | Adults/retrospective observational study | 2 years | Focal and generalised epilepsy | Not assessed | levetiracetam: 46.4; Oxcarbazepine: 41.2 |
| Ramsey RE et al., [23] | Adults, children/parallel, randomised, open label, multicenter trial | 6 months | Generalised tonic clonic seizures | Valproate:64; phenytoin:53 | Valproate:1.1; phenytoin:2 |
| Thilothammal N et al., [24] | Children/Randomised, double blind clinical trial | 2 years | Generalised tonic clonic seizures | Valproate:73.7; phenytoin:69.5 | Not assessed |
| Callaghan N et al., [25] | Adults, children/Randomised, double blind clinical study | 2 years | Focal and generalised epilepsy | valproate: 59.4; phenytoin: 73.0 | Not assessed |
| Present study Syed AA et al., 2018 | 10 years or older, male and female, cohort, observational study | 6 months | Focal and generalised epilepsy | Oxcarbazepine:100; Lacosamide:89; Levetiracetam:80; Phenytoin:75; Valproate:64 | Not assessed |
Therapeutic inefficacy refers to lack of effect and/or worsening of cases resulting into patient withdrawal from the study. 2-Seizure freedom refers to percentage of people without seizure at the end of the study
Quality of Life and Seizure Control
The influence of various factors on QOL was measured using the QOLIE-31 questionnaire. Earlier studies have proved female sex, matrimonial status; high illiteracy and agrarian habitation to be significantly related with a decreased QOL [12,26]. Our results depicted significant association between presence of ADR and low QOLIE scores but was not found to be statistically significant. Conversely, seizure control, seizure type, age, gender, alcohol intake were not associated with QOLIE scores and seizure control which was in contrast to a study showing seizure type, age and gender as significant predictors of QOL [27]. Results of this study showed that patients on Levetiracetam had the best QOL and phenytoin the least. On the other hand, there are no studies evaluating the predictors of seizure control. In this study, age was found to be significant predictor of seizure control in a multivariable logistic regression model.
Adverse Reactions to Antiepileptic Drugs
ADRs have been one of the important cause of drug discontinuation leading to therapeutic inefficacy [17,21,23]. Conversely, ADRs were not responsible for drug discontinuation in this study which may be due to mild nature of the ADRs. Sodium valproate was the drug having maximum number of ADRs followed by Levetiracetam, Phenytoin, Lacosamide and Oxcarbazepine. Levetiracetam was responsible for headache and irritability which was confirmed by another study. Similarly, rashes and hypersensitivity reactions with phenytoin seen in this study were also reported by other study [28].
Limitation
The present study has many limitations. Because the study was designed as a pilot study, sample size and duration of study was small. Only five drugs and two types of epilepsy patients were considered. The influence of comorbid conditions and duration of treatment was not considered while evaluating QOL. As majority of the patients were outpatients, verbal assessment of MMA scores was used to assess medication adherence. Only patients on monotherapy were included whereas polytherapy patients were completely excluded. Ideally the study would have been designed to detect the improvement in QOLIE scores from baseline, which was not possible due to short duration of the study.
Conclusion
In conclusion, levetiracetam had better efficacy and tolerability when compared to the other four drugs; and the lowest QOL was seen in patients on phenytoin. The results of the present study advocate that Levetiracetam is the first, Oxcarbazepine and Lacosamide, better and Sodium Valproate and Phenytoin are the last option as monotherapy in epileptic patients. Thus, effective seizure termination and improved QOL can be attained by striking a balance between the efficacy and harmful adverse effects of the drugs.