Serum Paraoxonase-Early Diagnostic Biomarker in NASH/NAFLD
Jyotchna Devi Bade1, Kiranmai Chittajallu2, Naazia Arifuddin3, Thejaswini Muppala4, Balachandrarao Naidu Menda5, Uma Lakshmi Valluri6
1 Assistant Professor, Department of Biochemistry, Great Easter Medical School and Hospital, Srikakulam, Andhra Pradesh, India.
2 Assistant Professor, Department of Biochemistry, Great Easter Medical School and Hospital, Srikakulam, Andhra Pradesh, India.
3 Clinical Biochemist, Department of Biochemistry, Prime Hospital, Hyderabad, Andhra Pradesh, India.
4 Assistant Professor, Department of Biochemistry, Great Easter Medical School and Hospital, Srikakulam, Andhra Pradesh, India.
5 Professor and Head, Department of Biochemistry, Great Easter Medical School and Hospital, Srikakulam, Andhra Pradesh, India.
6 Retires as Professor, Department of Biochemistry, Osmania Medical College, Hyderabad, Telangana, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Jyotchna Devi Bade, Flat No. 03, 786 Towers, Near Saraswati Mahal Theater, Bakarsahebpeta, Srikakulam-532001, Andhra Pradesh, India.
E-mail: jyotchna.appaji@gmail.com
Introduction
Non-Alcoholic Steatohepatitis (Nash)/Non-Alcoholic Fatty Liver Disease (NAFLD) is one of the important causes of morbidity and mortality worldwide. Serum paraoxonase (PON 1) is a glycosylated protein, an esterase associated with High density lipoprotein (HDL), and can be speculated to be a potential biomarker of liver injury or lipid peroxidation.
Aim
To assess the PON 1activity in NASH/NAFLD in combination with standard liver function tests in evaluating liver damage.
Materials and Methods
The case-control study was conducted at Department of Biochemistry, Osmania General Hospital, Hyderabad, India. Serum Paraoxonase levels were estimated in 30 clinically diagnosed NASH/NAFLD patients, and 30 apparently healthy blood donors. Serum Paraoxonase levels was measured by kinetic assay in spectrophotometer along with standard liver function tests (total and direct bilirubin, total protein, albumin, Alanine Transaminase (AST), Aspartate Transaminase (AST), Alkaline Phosphatase (ALP). Results were expressed as Mean±Standard deviation (Mean±SD) of various parameters. Paired t-test was used to compare the parameters between control and case group. ROC curve analysis was done to assess maximum sensitivity and maximum specificity and diagnostic efficiency. A p-value of <0.05 was considered to be statistically significant.
Results
Serum PON 1 levels were significantly (p-value < 0.001) decreased in patients (712.7±232.9 ng/mL) when compared with controls (988±238 ng/mL). ROC curve analysis showed increased senstivity (86.67%) and decreased specificity (60%) of PON 1 as compared to total and direct bilirubin, AST and ALT. The Area Under Curve (AUC) for serum PON 1 was 0.79, and PON 1 had highest diagnostic efficiency of 73% as compared to other liver parameters (56.6%-69.9%) in NASH /NAFLD.
Conclusion
Serum Paraoxonase is an early diagnostic marker for patients with oxidative stress like NASH/NAFLD and serum PON 1 levels should be measured along with standard liver function tests.
Antioxidant, Diagnostic efficiency, Liver damage, Liver function tests, Oxidative stress
Introduction
Alcoholic liver diseases, non-alcoholic fatty liver disease, viral hepatitis and hepatocellular carcinoma are the major primary diseases of liver [1]. Iron and copper overload, chronic alcohol consumption and non-alcoholic steatohepatitis also leads to damage due to cellular oxidative stress [2].
NAFLD is defined as accumulation of fat in the cytoplasm of hepatocytes when other causes of steatosis such as excessive alcohol consumption, drugs, Hepatitis B and C are ruled out [3]. The prevalence rate of NAFLD in Asia-pacific region is 12-24% [4]. Globally, the annual incidence in adult population was reported to be 3-5% [5]. The prevalence of NAFLD, with abnormal enzymes is influenced by the presence of co-existing obesity, diabetes and dyslipidemia [6].
Currently the available data suggested a two-hit model of pathogenesis encompassing of 2 sequential events:
Hepatic steatosis due to Insulin resistance
Hepatocyte inflammation and necrosis due to oxidative injury to hepatocytes.
This oxidative stress results in lipid peroxidation of lipid laden cells, the reactive end-products cause mitochondria and plasma membrane damage [7].
PON 1 is a glycosylated protein, an esterase with an apparent mass of 43-47 KDa and consists of 354 amino acids. In mammals, PON gene family has 3 members PON1, PON2 and PON3, located on chromosome 7 [8]. It is synthesised in the liver, secreted into plasma where it binds to HDL. HDL associated paraoxonase inhibits the biological activity of oxidised low density lipoprotein (ox-LDL) [9]. As an antioxidant, it hydrolyses lipid peroxides, and thus intervenes in the regulation of oxidative stress and hepatic cell apoptosis [10,11].
Based on these findings, the present study was designed to evaluate serum PON1 levels in NASH/NAFLD patients, and assess its diagnostic utility as an early biomarker.
Materials and Methods
The case-control study was conducted in the Department of Biochemistry, Osmania General Hospital, Hyderabad, India from May 2013 to December 2014. The present study is a part of the previously published work, ‘Serum Paraoxonase-a marker of alcoholic liver diseases’ [12]. Ethical committee approval was obtained from the Ethics Scientific Committee of Osmania Medical College (Reg No.:M120717031) before commencement of the study. The nature and purpose of the study was explained to the participants and informed written consent was obtained before collection of blood samples.
Sources of Sample
Department of Biochemistry, Osmania General Hospital.
Department of Medicine, Osmania General Hospital.
Department of Liver Care Unit, Osmania General Hospital.
Inclusion criteria: Clinically diagnosed 30 (power of the study is 80 and 95% CI) NASH/NAFLD patients admitted or attending the Outpatient Department was selected. Patients with Type II Diabetes Mellitus (T2DM) with duration of disease ≥10 years or obese individuals with grade II fatty liver infiltration confirmed with Ultrasound were included. Apparently healthy 30 individuals were recruited as controls.
Sample collection: Blood samples (5 mL) were collected in plain vacutainer by venepuncture while maintaining strict aseptic precaution. To obtain sera, samples were centrifuged at 3000 rpm for 10 minutes. A portion of the sera was used for analysis of liver function parameters such as total bilirubin, direct bilirubin, AST, ALT, ALP, total protein and albumin. The remaining sera were stored at -20°C for PON 1 analysis. Homolysed and lipemic samples were excluded.
Biochemical Parameters
The liver function test parameters: total bilirubin, direct bilirubin, AST, ALT, ALP, total proteins and albumin were measured by TransasiaChem 5 semi-autoanalyser using ErbaPATH reagents. Serum PON was estimated using 4-nitrophenylacetate (Sigma Aldrich Chemicals) as substrate. The rate of generation of 4-nitrophenol was determined at 402nm (402= 14000 M-1cm-1 at pH7.4) and kinetic curve were plotted [13].
Statistical Analysis
Results were expressed as Mean±SD. The data was analysed using Graph Pad Prism Demo software version 6. Paired t-test was used to assess the differences between various parameters of cases and control. ROC curve analysis was done to assess maximum sensitivity and maximum specificity, diagnostic efficiency and area under curve. A p-value <0.05 was considered to be statistically significant.
Results
Mean±SD of various standard liver function tests and serum PON 1 levels in cases and controls are presented in [Table/Fig-1]. Serum PON 1 levels were significantly (p-value <0.001) decreased in patients when compared with controls. Among LFT parameters, statistical difference (p-value<0.05) was observed between total bilirubin and albumin levels.
Mean±SD of various standard liver function tests and serum PON 1 levels in cases and controls.
| Parameters | Reference range | Controls | Cases | p-value |
|---|
| Total bilirubin (mg/dL) | 0.1-1.2 | 0.82±0.51 | 1.19±0.68 | 0.0204* |
| Direct bilirubin (mg/dL) | 0.0-0.3 | 0.36±0.31 | 0.42±0.32 | 0.4637 |
| AST (U/L) | 10-40 | 27.7±13.44 | 31.99±25.87 | 0.4235 |
| ALT (U/L) | 10-40 | 24.31±14.36 | 32.5±19.12 | 0.0657 |
| ALP (U/L) | 24-112 | 70.63±15.01 | 65.97±16.45 | 0.2564 |
| Total proteins (g/dL) | 6.0-8.3 | 7.21±0.96 | 6.76±1.2 | 0.1142 |
| Albumin (g/dL) | 3.2-5.0 | 4.3±0.72 | 3.8±0.91 | 0.0217* |
| Serum PON 1 (ng/mL) | - | 988±238 | 712.7±232.9 | <0.001* |
AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase;
PON 1: Paraoxonase; Students’ paired t-test; *p-value< 0.05-statistically significant
Sensitivity of PON 1 was 86.67% which was much higher than standard LFT parameters that ranged from 20% to 73.33%, whereas the specificity was lower (60%) than standard LFT parameters with the exception of total protein whose specificity was 50% [Table/Fig-2].
Sensitivity, Specificity and Best cut-off value (BCV), Area under curve (AUC), Diagnostic efficiency (DE) and significance (p-value) in discriminating cases and controls.
| Total bilirubin | Direct bilirubin | AST | ALT | ALP | Total protein | Albumin | PON 1 |
|---|
| Sensitivity | 60 | 36.67 | 46.67 | 36.67 | 20 | 70 | 73.33 | 86.67* |
| Specificity | 80 | 83.33 | 70 | 86.21 | 96.67 | 50 | 63.33 | 60 |
| BCV | >0.95 | >0.45 | >32.8 | >33.7 | <48.5 | <7.11 | <4.01 | <924.8 |
| AUC | 0.7111 | 0.5522 | 0.57 | 0.59 | 0.56 | 0.59 | 0.60 | 0.79* |
| DE | 69.9 | 56.6 | 58.3 | 59.9 | 58.3 | 59.9 | 68.3 | 73* |
| p-value | 0.0050* | 0.4871 | 0.32 | 0.20 | 0.36 | 0.21 | <0.018* | <0.0001 |
*p-value<0.05-statistically significant
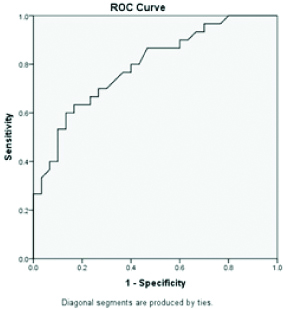
Area under curve is 0.79 highest for PON 1 when compared to standard LFT parameters (AUC=0.71 for total bilirubin, AUC=0.60 for albumin, AUC=0.59 alanine transaminase and total protein) indicating PON 1 has the diagnostic accuracy in NASH/NAFLD patients when compared to standard LFT parameters [Table/Fig-3,4,5,6,7 and 8].
ROC of serum paraoxonase 1.
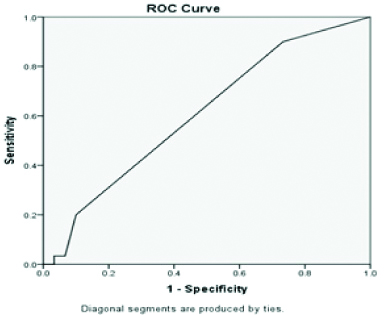
ROC of Aspartate transaminase.
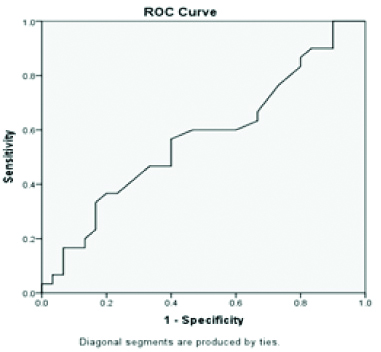
ROC of Alanine transaminase.
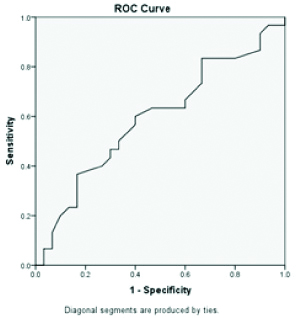
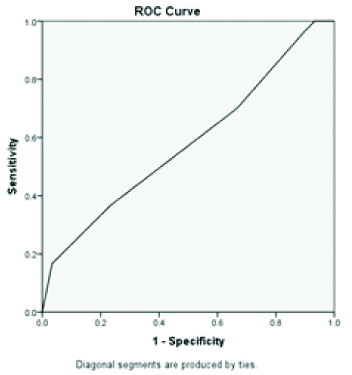
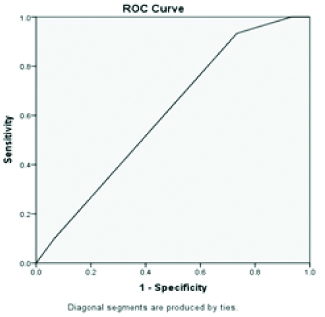
Discussion
In the absence of significant alcohol intake, viral infection, or any other specific aetiology of liver disease, when the fat accumulation exceeds more than 5% of hepatocytes then it is termed NAFLD. Histologically, it ranges from Simple steatosis(SS) to NASH, which is characterised by SS+ necroinflammation [14]. Clinico-pathological diagnosis of NASH requires presence of cell injury seen as cellular ballooning, focal necrosis, fibrosis and inflammation and exclusion of alcohol as major contributor [15]. The common predisposing factors are diabetes and central obesity while Insulin Resistance (IR) is detectable universally [16]. Two important key factors in development and progression from steatosis to more advanced stages of liver damage are oxidative stress and lipid peroxidation [10]. Oxidative stress arises due to imbalance between reactive metabolites and antioxidant defence system. These reactive metabolites induce free radical mediated lipid peroxidation that regulates the progression of liver diseases [17]. Morphological changes are observed in mitochondria, which include swelling and intramitochondrial crystals, indicating presence of pro-oxidant free radicals (superoxide and hydroxyl radicals) [18,19].
Liver is the major site of HDL synthesis and has highest PON1 gene expression [20-21]. Analysis by PON 1 inhibitors shows that all phases of LDL oxidation and its initiation (conjugated dienes formation), propagation (peroxides formation), and decomposition (aldehydes formation) conferred by PON1 are affected [22]. PON 1 degrades specific ox-LDL and prevents the generation of lipoperoxides during LDL oxidation. PON1 inhibits HDL oxidation and preserves its function [23].
Based on the available literature and established facts, the present study was done to determine the diagnostic utility of PON 1 is an early indicator of NAFLD and liver damage due to oxidative stress. The diagnosis of NAFLD based on asymptomatic elevation of aminotransferases, radiological findings of fatty liver or unexplained persistent hepatomegaly. Although serum AST and ALT are raised up to 2-3 times the normal level in NAFLD, Mofrad P et al., reported normal ALT levels in NAFLD therefore these values are not reliable for diagnosis of NASH/NAFLD [24,25].
In the present study, PON 1 levels were significantly decreased due to oxidative stress even when the standard liver parameters remained the same, indicating that sensitivity of PON1 as an early marker. In addition, the diagnostic efficiency of PON 1 was much higher than all the standard liver parameters collectively.
Atamer A et al., evaluated the changes in oxidative stress in hepatosteatosis patients in terms of lipid peroxidation, nitric oxide and PON 1 activity, and reported that PON1 activity and nitric oxide levels were significantly lower (p-value <0.001) in patients with hepatosteatosis when compared with the controls. They also reported that the levels of LDL were significantly raised and that of HDL and apolipoprotein A1 were significantly lower without any changes in levels of hepatic enzymes thus indicating that changes in lipid metabolism occurs in hepatosteatosis that are important indicators of liver cell impairment [26]. Bell LN and Theodorakis JL, reported similar results, where success rate of diagnosis of NAFLD patients using PON 1 was 100% with an area under ROC=1.0, as compared to the success rate of ALT which was 55% with an area under ROC=0.53 [27].
The decreased levels of PON 1 observed could be due to alteration in PON1 synthesis by liver, alterations in HDL structure or it is alterations in HDL structure [28-30].
Routinely done standard biochemical tests for liver dysfunction are not a reliable indicator of the presence or absence of liver disease, therefore, histopathological examination of liver via biopsy has become the diagnostic method of choice. However, liver biopsy is subjected to sampling bias and has several disadvantages like invasive nature, perspicacity and a mortality rate which is noteworthy. Numerous studies have reported that the estimation of serum paraoxonase along with standard LFT will improve the clinical outcome by assessing the extent of liver damage [11,31,32].
Limitation
Patients with T2DM were taken as cases, but T2DM itself is an independent risk factor for altered PON 1 value, so, it’s hard to determine whether the altered PON 1 values are a result of the combined effect of T2DM and NASH/NAFLD or NASH/NAFLD alone or T2DM alone. For implementing in routine assay it is better to adapt non-toxic lactonase assay rather than the use of toxic and unstable substrates.
Conclusion
From the present study, it was established that detection of serum PON 1 levels can be used as an early, non-invasive diagnostic marker in addition to standard liver parameters, while liver biopsy still remains the gold standard for diagnosing NASH/NAFLD.
AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase;
PON 1: Paraoxonase; Students’ paired t-test; *p-value< 0.05-statistically significant
*p-value<0.05-statistically significant
Author Declaration:
Financial or Other Competing Interests: No
Was Ethics Committee Approval obtained for this study? Yes
Was informed consent obtained from the subjects involved in the study? Yes
For any images presented appropriate consent has been obtained from the subjects. NA
PLAGIARISM CHECKING METHODS: [Jain H et al.]
Plagiarism X-checker: Jul 13, 2019
Manual Googling: Aug 08, 2019
iThenticate Software: Sep 18, 2019 (15%)
[1]. Lim YS, Kim WR, The global impact of hepatic fibrosis and end-stage liver diseaseClin Live Dis 2008 12:73310.1016/j.cld.2008.07.00718984463 [Google Scholar] [CrossRef] [PubMed]
[2]. Cahill A, Cunningham CC, Adachi M, Ishii H, Bailey SM, Fromenty B, Effects of alcohol and oxidative stress on liver pathology: the role of the mitochondrionAlcohol Clin Exp Res 2002 26(6):907-15.10.1111/j.1530-0277.2002.tb02621.x12068261 [Google Scholar] [CrossRef] [PubMed]
[3]. Angulo P, Lindor KD, Non-alcoholic fatty liver diseaseJ Gastroenterol Hepatol 2002 17(Suppl):S186-90.10.1046/j.1440-1746.17. s1.10.x12000605 [Google Scholar] [CrossRef] [PubMed]
[4]. Farrell GC, Chitturi S, Lau GK, Sollano JD, Asia-Pacific Working Party on NAFLD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summaryJ Gastroenterol Hepatol 2007 22(6):775-77.10.1111/j.1440-1746.2007.05002.x17565629 [Google Scholar] [CrossRef] [PubMed]
[5]. Lazo M, Clark JM, The epidemiology of nonalcoholic fatty liver disease: a global perspectiveSemin. Liver Dis 2008 28:339-50.10.1055/s-0028-109197818956290 [Google Scholar] [CrossRef] [PubMed]
[6]. Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M, Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patientsAm J Gastroenterol 1999 94:3010-14.10.1111/j.1572-0241.1999.01451.x10520861 [Google Scholar] [CrossRef] [PubMed]
[7]. Theise ND, Histopathology of alcoholic liver diseaseClin Liver Dis 2013 2:64-67.10.1002/cld.17230992826 [Google Scholar] [CrossRef] [PubMed]
[8]. Clendenning JB, Humbert R, Green ED, Wood C, Traver D, Furlong CE, Structural organisation of the human PON1 geneGenomics 1996 35:586-89.97.10.1006/geno.1996.04018812495 [Google Scholar] [CrossRef] [PubMed]
[9]. Aviram M, Billecke S, Sorenson R, Bisgaier C, Newton R, Rosenblat M, Paraoxonase active site required for protection against LDL oxidation involves its free sulfhydryl group and is different from that required for its arylesterase/paraoxonase activities: selective action of human paraoxonaseallozymes Q and RArterioscler Thromb Vasc Biol 1998 18:1617-24.10.1161/01.ATV.18.10.16179763535 [Google Scholar] [CrossRef] [PubMed]
[10]. Videla LA, Rodrigo R, Araya J, Poniachik J, Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver diseaseFree Radic Biol Med 2004 37:1499-507.10.1016/j.freeradbiomed.2004.06.0331545429017850780 [Google Scholar] [CrossRef] [PubMed] [PubMed]
[11]. Camps J, Marsillach J, Joven J, Measurement of serum paraoxonase-1 activity as a potential biomarker for chronic liver impairmentClin Chim Acta 2007 386:114-15.10.1016/j.cca.2007.07.01617850780 [Google Scholar] [CrossRef] [PubMed]
[12]. Devi BJ, Lakshmi VU, Arifuddin N, Rao JR, Ch. Kiranmai, Serum paraoxonase-Marker of alcoholic liver cirrhosisInt J Clin Biochem Res 2018 5(4):513-16.10.18231/2394-6377.2018.0109 [Google Scholar] [CrossRef]
[13]. Haagen L, Brock A, A new automated method for phenotypingarylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenylacetateEur J Clin Chem Clin Biochem 1992 30(7):391-95.10.1515/cclm.1992.30.7.391 [Google Scholar] [CrossRef]
[14]. Argo CK, Caldwell SH, Epidemiology and natural history of non-alcoholic steatohepatitisClin. Liver Dis 2009 13:511-31.10.1016/j.cld.2009.07.00519818302 [Google Scholar] [CrossRef] [PubMed]
[15]. Baumeister SE, Völzke H, Marschall P, John U, Schmidt CO, Flessa S, Impact of fatty liver disease on health care Utilization and costs in a general population: a 5-year observationGastroenterology 2008 134:85-94.10.1053/j.gastro.2007.10.02418005961 [Google Scholar] [CrossRef] [PubMed]
[16]. Agarwal SR, Malhotra V, Sakhuja P, Sarin S, Clinical biochemical and histological profile on non-alcoholic steatohepatitisIndian J Gastroenterol 2001 20:183-86. [Google Scholar]
[17]. Loguercio C, Federico A, Oxidative stress in viral and alcoholic hepatitisFree Radic. Biol. Med 2003 34(1):1-10.10.1016/S0891-5849(02)01167-X [Google Scholar] [CrossRef]
[18]. Pessayre D, Fromenty B, NASH: A mitochondrial diseaseJ Hepatol 2005 42:928-40.10.1016/j.jhep.2005.03.00415885365 [Google Scholar] [CrossRef] [PubMed]
[19]. Caldwell SH, de Freitas LA, Park SH, Moreno ML, Redick JA, Davis CA, Intramitochondrial crystalline inclusions in non alcoholic steatohepatitisHepatology 2009 49:1888-95.10.1002/hep.2285119274750 [Google Scholar] [CrossRef] [PubMed]
[20]. Mackness MI, Arrol S, Abbott C, Durrington PN, Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonaseAtherosclerosis 1993 104:129-35.10.1016/0021-9150(93)90183-U [Google Scholar] [CrossRef]
[21]. Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, Protective effect of high-density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low-density lipoproteinJ Clin Invest 1995 96:2882-91.10.1172/JCI1183598675659 [Google Scholar] [CrossRef] [PubMed]
[22]. Primo-Parmo SL, Sorenson RC, Teiber J, La Du BN, The human serum paraoxonase/arylesterase gene (PON1) is one member of a multigene familyGenomics 1996 33:498-507.10.1006/geno.1996.02258661009 [Google Scholar] [CrossRef] [PubMed]
[23]. Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN, Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonaseJ Clin Invest 1998 101:1581-90.10.1172/JCI16499541487 [Google Scholar] [CrossRef] [PubMed]
[24]. Khoonsari M, Azar MMH, Ghavam R, Hatami K, Asobar M, Gholami A, Clinical manifestations and diagnosis of Nonalcoholic Fatty Liver DiseaseIran J Pathol 2017 12(2):99-105. [Google Scholar]
[25]. Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Clinical and histological spectrum of nonalcoholic fatty liver disease associated with normal ALT valuesHepatology 2003 37:1286-92.10.1053/jhep.2003.5022912774006 [Google Scholar] [CrossRef] [PubMed]
[26]. Atamer A, Bilici A, Yenice N, Selek S, Ilhan N, Atamer Y, The importance of Paraoxonase 1 activity, nitric oxide and lipid peroxidation in hepatosteatosisJournal of International Medical Research 2008 36:77110.1177/14732300080360041918652773 [Google Scholar] [CrossRef] [PubMed]
[27]. Bell LN, Theodorakis JL, Serum proteomics and biomarker discovery across the spectrum of non-alcoholic fatty liver disease. American Association for the Study of Liver DiseasesWiley Inter ScienceDOI 10.1002/ hep.23271 [Google Scholar]
[28]. Deakin S, Leviev I, Gomaraschi M, Calabrese L, Franceschini G, James RW, Enzymatically active paraoxonase-1 is located at the external membrane of producing cells and released by a high affinity, saturable, desorption mechanismJ Biol Chem 2002 277:4301-08.10.1074/jbc.M10744020011726658 [Google Scholar] [CrossRef] [PubMed]
[29]. James RW, Deakin SP, The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activityFree Rad Biol Med 2004 37:1986-94.10.1016/j.freeradbiomed.2004.08.01215544917 [Google Scholar] [CrossRef] [PubMed]
[30]. Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, Human serum paraoxonase (PON1) is inactivated by oxidized low density lipoprotein and preserved by antioxidantsFree Radical Biol Med 1999 26:892-904.10.1016/S0891-5849(98)00272-X [Google Scholar] [CrossRef]
[31]. Ferré N, Camps J, Prats E, Vilella E, Paul A, Figuera L, Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damageClin Chem 2002 48:261-68. [Google Scholar]
[32]. Marsillach J, Ferré N, Vila MC, Lligoña A, Mackness B, Mackness M, Serum paraoxonase-1 in chronic alcoholics: Relationship with liver diseaseClin Biochem 2007 40:645-50.10.1016/j.clinbiochem.2007.01.020 [Google Scholar] [CrossRef]