Rapid nucleic acid amplification techniques such as PCR help in identification of M. tuberculosis in clinical specimens [3]. While AFB requires 104-106 bacilli/mL of tissue or fluid specimens for accurate smear diagnosis, PCR can detect the presence of M. tuberculosis in early stages when bacilli concentration is as low as 10 bacilli/mL [12]. For definitive mycobacteriological detection, targeted PCR assays can be used for amplification of various gene segments such as IS6110 element 5 and mpt646 and the 64kDa protein encoding gene 4, which considerably reduced the processing time of samples in diagnostic laboratory [13]. Early diagnosis of FGTB is essential for prompt management, treatment and prevention of complications of FGTB such as fibrosis and infertility. Rapid diagnosis using PCR, prompt anti-tuberculous treatment and appropriate surgical interventions can improve the results in terms of management of disease, restoration of female reproductive functions and reduction of procedural and postoperative complications [14]. Although various studies have been conducted till date on utility of TB–PCR for diagnosis of FGTB in addition to AFB staining and culture in India. Our study may add to the present knowledge regarding improvement in rapid detection rate of FGTB and uncover diagnostic dilemma in FGTB cases leading to infertility. With this objective, the present study was undertaken to assess the diagnostic performance of PCR using IS6110 in infertility due to GTB.
Materials and Methods
The present cross-sectional study was conducted over a period of two years from October 2013 to October 2015 at Microbiology Department of Tertiary Care Hospital, Aurangabad, Maharashtra, India. Women with unexplained infertility attending the infertility clinic of Obstetrics and Gynaecology Department were involved in the study after obtaining the institutional ethical committee approval (ECR/314/Inst/MH/2013). The purpose of the study was explained to all the participants and informed consent was obtained before commencement of the study.
The socio-demographic information, risk factors and clinical symptoms were recorded using a structured questionnaire, and 50 infertile women who met inclusion and exclusion criteria were enrolled for the study.
Inclusion criteria: Women aged 18-40 years, did not conceive after more than 12 month of regular intercourse without using any contraceptive, and their male partner sperm count within normal limit. Participant with one live birth or abortion but failed to conceive for second time child after 12 month of regular intercourse without using any contraceptive or participant suspected of having GTB on clinical grounds were also included.
Exclusion criteria: Participant aged less than 18 and above 40 years using contraceptive, or with low sperm count of male partner. Participants with presence of genital anomaly (either in participant or her husband), ovarian or uterine hormonal imbalance, galactorrhea, hirsutisum, diabetes, hypertension, thyrotoxicosis, malignancy of female genital tract or other proven clinical cause of infertility were excluded.
Relevant clinical specimens like endometrial biopsy were collected in normal saline and endometrial aspirate, menstrual blood were collected in sterile vials using standard collection procedures. Endometrial biopsy was taken from both cornual ends [2]. Menstrual blood was taken on first day of menstruation and was evaluated by AFB staining, culture and TB-PCR [15].
For microscopic examination of acid fast bacilli, the homogenised concentrated smeared sample was stained with Ziehl Neelsen (ZN) stain [16]. The samples were cultured on L J medium and the growth of M. tuberculosis observed [16]. Because the specimens are less likely to be contaminated with other microorganisms, they were homogenised and inoculated directly onto culture media without the use of a decontaminating solution [17].
For Polymerase Chain Reaction Analysis
Processing of sample:Endometrial biopsy and aspirate: Fine mincing done against the wall of the container; the minced tissue was suspended in 1 mL of sterile distilled water and centrifuged at 1000 rpm for 1 minute to sediment large particles. About 500 μL of supernatant was taken and centrifuged at 8000 rpm for 10 minutes. The supernatant was decanted and the pellet was re-suspended in 250 μL of Lysis Buffer 1.
Menstrual blood: To 200 μL of menstrual blood 20 μL of Proteinase K was added, it was mixed by vortexing for 15 seconds, and following it DNA was extracted.
DNA extraction: DNA was extracted according to the manufacturer guidelines by using GeNeiTM DNA extraction reagent set for Mycobacterium tuberculosis kit (Catalogue No.:610670300011730, Revision No.00311210, Merck) procured from BangloreGenei, India.
DNA amplification: Amplification of M. tuberculosis DNA done with the help of GeNeiTM Amplification Reagent set for Mycobacterium tuberculosis kit (Catalogue No:610670300011730, Revision No.00311210, Merck) procured from Bangalore Genei, India. Precautions were taken to avoid false positivity. Preparation of PCR reagents, addition of template DNA and analysis of amplified products were done in three different rooms to avoid carryover contamination. Reagents were aliquoted and each aliquot was used only once. DNAs from the samples were amplified using following custom synthesised primers from Banglore GeNei, Banglore, India that were proprietary to the company.
IS6110a (5’ – CCT GCG AGC GTA GGC GTC GG – 3’)
IS6110b (5’ – CTC GTC CAG CGC CGC TTC GG – 3’)
First amplification: Total number of amplification master mix 1 was calculated and 9μl of amplification master mix 1 was then aliquoted into required number of labeled vials. Then 3 μL DNA sample was added to the respective labeled vials; 3μL of positive control DNA to the positive control vial and last vial with no DNA sample was set-up as reagent control. First PCR amplification was performed using Applied Biosystems GeNeAmpTM, MJ Reseach and Biorad thermal cyclers. The cycling parameter used was initial denaturation at 94°C for 5 minutes, followed by denaturation at 94°C for 30 seconds, annealing at 68°C for 1 minute, extension at 72°C for 1 minute for 20 cycles and a final extension at 72°C for 7 minutes. The amplified samples were then stored at 4°C.
Nested amplification: As per manual instructions, 15 μL of amplification master mix 2 was added to each tube after the first PCR was completed, and contents of the vials were mixed by gentle tapping. The cycling parameter used was initial denaturation at 94°C for 15 minutes, followed by denaturation at 94°C for 30 seconds, annealing at 68°C for 1 minute 30 seconds, extension at 72°C for 1 minute 30 seconds with 30 cycles and a final extension at 72°C for 10 minutes. The amplified products were stored at 2-8°C.
Analysis of Amplified Product was done by agarose gel electrophoresis (2.5%) at 100-120 volts for 20-25 minutes. Gel was stained with ethidium bromide and viewed under UV transilluminator.
Interpretation of ResultAn amplification product of size 123 bp is indicative of M. tuberculosis complex.
An amplification product of internal control DNA is 340 bp
Positive: band at 123 bp and 340 bp/band at only 123 bp.
Negative: band at 340 bp
Repeat the DNA extraction: No band
Absence of 340 bp band indicates that sample contains inhibitors or the DNA extraction failed.
Statistical Analysis
Descriptive statistics for all the categorical clinical parameters were presented as frequency and percentage. Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 21. Sensitivity for TB PCR assay and AFB smear for the diagnosis of probable and confirmed TB were calculated by considering the AFB culture as separate gold standard. Categorical variables were compared using Chi-Square test. A p-value <0.05 was considered as statistically significant.
Results
All the 50 samples collected from females presented with infertility and suspected to have FGTB for M. tuberculosis were assessed by three different diagnostic methods namely ZN staining, culture on LJ medium and TB PCR. If any of the three investigating parameters was positive it is taken as evidence of FGTB. Although affection of endometrium usually indicates the involvement of fallopian tubes too, the samples were collected from endometrium due to ease of procedure.
Out of 50 infertile females studied; majority i.e., 28 (56%) patients belonged to 21-25 years age group followed by 15 (30%) to 26-30 years age group; 4 (8%) were less than or equal to 20 years of age and least i.e., 3 (6%) were from 31-35 years age group. Almost 36 (72%) patient presented with primary infertility and 14 (28%) patient had secondary infertility.
Specimens play an important role in the accuracy of detection of FGTB. About 34 (68%) of clinical specimens collected were endometrial biopsies followed by 12 (24%) menstrual fluid sample and least 4 i.e., (8%) were endometrial aspirates. In majority of patients 39 (78%), the sole presenting symptom was either primary or secondary infertility, while 8 (16%) patients clinically presented with menstrual disturbances with infertility either in form of oligomenorhhea (6), polymenorrhhea (1), amenorhhea (1), while 2 (4%) presented with abdominal pain and only one (2%) patient presented with abdominal mass with infertility.
Out of 50 clinical specimen examined, 2 (4%) of endometrial biopsies were positive by ZN microscopy whereas 1 (2%) of endometrial biopsy were positive by culture on LJ medium [Table/Fig-1,2]. Among these 2 endometrial biopsies positive on ZN microscopy; only 1 (2%) was also positive on PCR in addition to culture and the other 1 was positive on ZN microscopy only [Table/Fig-3]. In the present study, PCR has failed to detect one case, which was positive by ZN staining.
Acid fast bacilli on ZN staining of endometrial specimen.
LJ medium showing growth of M. tuberculosis.
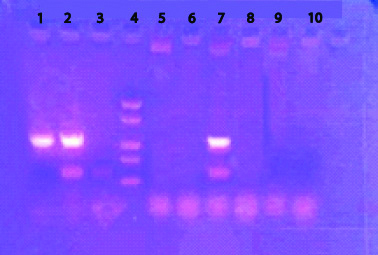
Gel elecrophoresis results of nested PCR.
1=Internal Contol (IC); 2=Positive Control (PC); 3=Negative Control (PC); 4=DNA Ladder size (100 bp); 5,6,8,9 & 10=Negative Sample (NS); 7=Positive Sample (PS).
All the 4 i.e., 8% endometrial aspirates and all the 12 i.e., 24% of endometrial blood specimens were negative by microscopy and culture on LJ medium. All the 50 clinical specimens were subjected to nested PCR assay targeting the IS1660 gene and 4 i.e., 8% of endometrial biopsies were positive by TB–PCR. All the 4 i.e., 8% of endometrial aspirates were negative and one i.e., 2% of menstrual blood were positive by TB-PCR. Difference between positivity for TB-PCR in endometrial biopsy and menstrual blood was not found to be statistically significant [Table/Fig-4].
Association of TB-PCR positivity in Endometrial biopsy and Menstrual Blood.
| TB-PCR (n-5) | Endometrial biopsy (n-34) | Menstrual blood (n-12) |
|---|
| TB-PCR positive | 4 (11.76%) | 1 (8.33%) |
| TB-PCR negative | 30 (88.24%) | 11 (91.67%) |
χ2 0.08; p-value >0.05
We studied distribution of positivity of TB-PCR in patients of primary and secondary infertility groups. It was seen that out of 36 patients of primary infertility only 4 (11.11%) were having TB-PCR positive. While out of 14 patient of secondary infertility; 1 (7.14%) were positive for TB-PCR. Difference between positivity for TB-PCR in primary and secondary infertility was not statistically significant [Table/Fig-5].
Association of TB-PCR positivity in primary and secondary infertility.
| TB-PCR | Primary infertility (n-36) | Secondary infertility (n-14) |
|---|
| TB-PCR positive | 4 (11.11%) | 1 (7.14%) |
| TB-PCR negative | 32 (88.89%) | 13 (92.86%) |
χ2 0.08; p-value >0.05
GTB is secondary to other TB of body (primarily, the lungs), Primary GTB is extremely rare. Out of 5 TB-PCR positive patients 3 (60%) patients gave history of TB either pulmonary or extra pulmonary in past or history of contact with TB patients. Association between TB–PCR positivity and past history of TB was found to be statistically significant (Chi-square -0.08, p-value <0.05).
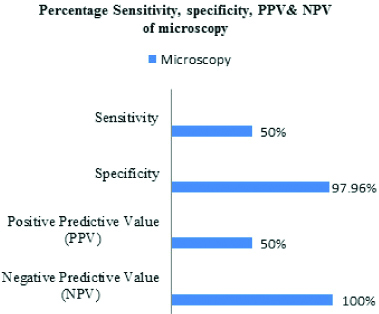
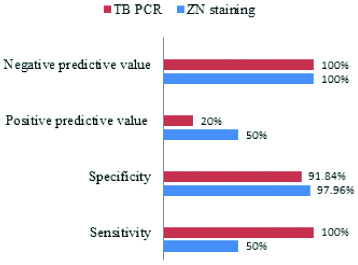
Considering culture as gold standard to diagnose FGTB, sensitivity and specificity of microscopy to diagnose FGTB was found to be 50% and 97.96% respectively, whereas sensitivity and specificity of TB-PCR was found to be 100% and 91.84%, respectively [Table/Fig-6,7]. The combined sensitivity and specificity of ZN staining and PCR considering culture as the gold standard are represented in [Table/Fig-8].
Comparison of microscopy and culture results.
| Microscopy | Culture | Total |
|---|
| Positive cases | Negative cases |
|---|
| Microscopy-positive cases | 1 (100%) | 1 (2.04%) | 2 |
| Microscopy-negative cases | 0 (0%) | 48 (97.96%) | 48 |
| Total | 1 | 49 | 50 |
 |
Comparison of TB-PCR and Culture results.
| TB-PCR | Culture | Total |
|---|
| Positive cases | Negative cases |
|---|
| TB-PCR positive cases | 1 (100%) | 4 (8.16%) | 5 |
| TB-PCR negative cases | 0 (0 %) | 45 (91.84%) | 45 |
| Total | 1 | 49 | 50 |
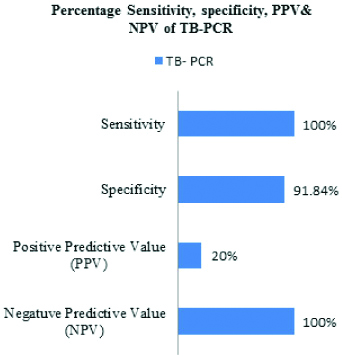 |
Comparision of sensitivity, specificity, positive and negative predictive value of ZN staining and PCR considering culture as gold standard.
Evidence of TB was found in 6 (12%) cases out of 50, by using all the investigative parameters alone or in combination [Table/Fig-9].
Distribution of study subjects positive for evidence of TB based on different diagnostic tests.
| Cases of evidence of TB | Infertility | Microscopy | Culture | TB-PCR |
|---|
| 1 | Primary | + | + | + |
| 2 | Primary | - | - | + |
| 3 | Primary | - | - | + |
| 4 | Primary | - | - | + |
| 5 | Secondary | - | - | + |
| 6 | Primary | + | - | - |
Discussion
In our study, majority of patient i.e., 43 (86%) were from the age group of 21-30 years, since at this age, after puberty the blood supply to the pelvic organs increases, and more bacilli could reach and infect the reproductive organs. These findings were in accordance with other studies which reported that 82% and 83.3% of patients of FTGB presented in the age group of 21-30 years in their study [18,19].
Specimens play an important role in the accuracy of detection of FGTB. In the present study, three different specimen types such as endometrial biopsy, endometrial aspirate and menstrual blood were collected from women suspected to have GTB. Of the 34 endometrial biopsies studied, 4 (11.76%) were positive for the evidence of FGTB. Out of 4 (11.76%) positive endometrial biopsies 1 (25%) was positive by all the three investigating parameters i.e., AFB staining, culture on LJ medium and TB PCR. Other three were positive by TB-PCR only. These findings are nearly similar to another study that reported 13% positivity by TB–PCR in endometrial biopsy specimens [18]. However, some studies report higher rates of detection such as 36.7% and 53.3% positivity by TB–PCR in endometrial biopsies [19,20]. Out of 12 menstrual blood samples studied in our study 1 (8.3%) was found to be positive on TB-PCR. The low rate of PCR positivity in menstrual blood and endometrial aspirate as compared to endometrial biopsy can be accounted to small sample size.
Of the total 34 endometrial biopsies studied, 4 (11.76%) and of 12 menstrual blood 1 (8.3%) were positive for the evidence of FGTB. Endometrial biopsy material is usually obtained after an invasive procedure. Therefore, a non-invasive way of getting samples for use in PCR is beneficial. One such material, when available could be menstrual blood. Our results showed that, though the detection of M. tuberculosis by PCR was higher in endometrial biopsy specimen, PCR was also found in menstrual blood but the difference in positivity was statistically insignificant. Proper sampling and if possible repeat sampling from the patient will enhance the sensitivity of PCR and thus improve the diagnostic yield in FGTB cases.
GTB is mostly secondary to TB of other parts of body such as lungs, kidneys, gastrointestinal tract, and often bones and joints. Primary FGTB is extremely rare [21]. Out of 5 TB-PCR positive patients 3 (60%) patient given history of tuberculosis either pulmonary 2 (50%) or extra-pulmonary (i.e., abdominal TB 1 (25%). Association between past history of tuberculosis and TB-PCR positivity, was found to be statistically significant. In this study evaluating 50 infertile women, 2 (4%) endometrial biopsies were found positive by ZN staining. However endometrial aspirates and menstrual blood were negative. Some studies reported that accurate diagnosis of GTB by ZN staining could be achieved in 1%, 1.6%, and 5.6% cases suspected to have FGTB [18,20,22]. FGTB mostly being Paucibacillary in earlier stages justifies the low detection rate of ZN staining in suspected FGTB cases.
Even though culture remains the gold standard in accurate identification of Mycobacterium when cultured; in the present study 1 (2%) endometrial biopsy was positive for growth on LJ medium. Due to paucibacillary nature, detection efficacy of GTB of smears and cultures are relatively low. These results were in concordance with various other studies which with rate of detection of 3.2%, 4.6%, 5.6% in suspected cases of FGTB [19,20,22]. However, in the current study culture was negative in all patients. This could be because of large number of bacteriologically silent lesions in genital tract tuberculosis that have paucibacillary nature.
The nested PCR assay targeting the IS1660 gene was positive in 10% (5 out of 50) of the patients. Comparison of the percentage of sensitivity for detection of mycobacteria by the three methods showed that TB-PCR assay has the highest sensitivity 100% whereas AFB detection by microscopy showed a sensitivity of 50%. This emphasises the accuracy and potential of PCR assay as a rapid diagnostic aid for routine use in the future. However, PCR failed to detect one case, which was positive by ZN staining. This false negative result of PCR could be due to Paucibacillary nature of the specimen, or the portion of the specimen taken for PCR might not have had any M. tuberculosis or the analysed specimen may contain inhibitors of PCR. Moreover, positivity depends on both proper sample collection from correct site and presence of TB infection in tested portion of sample. Furthermore, PCR false positives were ruled out, since all negative controls remained negative in each batch of PCR. The clinical history and supporting laboratory investigations were also recorded, that rules out the unlikelihood of misdiagnosis due to false positive results of PCR.
Limitation
The possibilities of carryover contamination of ZN smear cannot be excluded as the specimen was non-cultivable for mycobacteria on culture on LJ medium and repeat TB-PCR. In certain circumstances, a gene with low copy number could not be detected, inspite of improvement and standardisation in PCR. Hence, further standardised PCR protocols are required for detection of other FGTB associated genes.
Conclusion
Our results demonstrate that PCR has highest sensitivity 100% with negative predictive value of 100% as compared to microscopy. PCR is rapid and most sensitive method of diagnosing FGTB as it is mostly Paucibacillary infection. Even culture may be negative in some cases as most of the infections are bacteriologically mute. Therefore, when the clinical suspicion is high, and smear and culture results are negative, PCR is the method of choice for identifying the infection in FGTB cases.
χ2 0.08; p-value >0.05
χ2 0.08; p-value >0.05