Magnesium (Mg) is the second most abundant intracellular cation and is vital for a multitude of cellular functions and enzymes. It acts as a catalytic co-factor for numerous biochemical reactions in the body, as a regulator of ion channels by stabilisation of cellular membranes, and is essential for various metabolic pathways such as glycolysis, oxidative phosphorylation, including protein and nucleic acid synthesis [1]. It is therefore responsible for a plethora of important biological functions including regulation of nerve impulse conduction, muscular contraction, cardiovascular function, bone matrix formation, and many other metabolic reactions.
Low Mg levels have been found to have a direct relationship with the risk of (T2DM), poor glycemic control, and IR [2,3]. Low Mg levels also promote inflammation, leading to an increased level of systemic inflammatory markers, and predisposing to atherosclerosis and hypertension [4,5]. Its deficiency can lead to varied central nervous system effects, and Mg treatment has been explored for rapid recovery from major depressive disorders [6,7]. Mg deficiency also produces osteoporosis by acting on formation and impacting the secretions of the parathyroid gland [8]. A derangement in its metabolism manifests with multisystem problems and Mg deficiency is, therefore, a significant clinical entity.
Worldwide, and especially in developing countries like India, Mg remains an ‘orphan’ nutrient. Most research pertaining to Mg status and deficiency are relatively old, and studies are particularly scarce in the Indian subcontinent. Mg remains an overlooked micronutrient, unlike iron or calcium, which have been well studied and are even supplemented antenatally to pregnant women. Several studies conducted in the western countries reported that the population consumed less than the required amount of Mg in food and that a significant percentage of them was hypomagnesaemic [9,10]. A Report of the Expert Group of the ICMR (2010) estimates the daily requirements of Mg to be 340 mg/day for adults.
An Indian study conducted amongst pregnant women in a rural setting showed that 43.6% of the subjects were hypomagnesaemic [14]. Dietary monitoring in pregnant women is of the utmost importance, and a shift towards processed foods with diminished micronutrient densities is suggestive of a dangerous trend towards deficiency, even in the urban population.
This study aimed to measure maternal and cord blood Mg levels at delivery and calculated the prevalence of hypomagnesaemia in pregnant women. Maternal and cord blood Mg levels were also correlated with other important maternal and foetal parameters, including the relation between Mg levels and period of gestation, birth weight, maternal dietary preference, gravidity, and parity.
Materials and Methods
This cross-sectional correlational study was conducted in a 1000-bedded tertiary care teaching hospital in Northern India. Due to a scarcity of prior prevalence studies, the forecasted prevalence of hypomagnesaemia was taken to be 50% to obtain the maximum possible sample size. Taking error to be 10% and alpha to be 0.05 assuming 95% Confidence Interval, the minimum sample size was 96. Our study population consisted of pregnant women (n=110) reporting for delivery to the labour room from May to August 2018. Informed consent was obtained from all the participants, and the Institutional Ethics Committee (IEC) approval (No. IEC BHDC/01 of 2018) was obtained before conducting the study.
Sample Collection and Laboratory Analysis
Venous blood from the mother was collected soon after establishing venous access in labour room during delivery and cord blood from the umbilical vein from newborn baby were collected within 5-10 minutes of delivery. Blood samples were immediately sent to the laboratory for analysis and analysed on the same day. Total serum Mg level was determined for maternal and cord blood. Blood samples were collected in plain (red) vacutainers of BD. The analysis was performed using a Fully Automated Biochemistry Analyser, EM360, Transasia. Maternal Mg level ≤1.8 mg/dL was taken to signify hypomagnesaemia [14].
Patient Information Proforma
Relevant clinical details were obtained during safe confinement post-delivery, including complications during pregnancy, antenatal dietary supplementation (Mg) if any, any drug therapy, etc. Details were collected from the case sheets and antenatal notes of each subject. Details such as dietary preference and socioeconomic information were also collected. Lacto-ovo vegetarians were placed in the vegetarian group and meat eaters were placed in the non-vegetarian group. The period of gestation was calculated using the date of the last menstrual period and the date of delivery. The weight of the newborn was recorded at birth.
Inclusion and Exclusion Criteria
Pregnant women of all age groups and gravidity with period of gestation ≥28 weeks reporting for delivery were included in our study. Singleton pregnancies with no foetal anomalies and cephalic presentation were included. Subjects that received Magnesium sulphate therapy, subjects on Mg supplements, or drug therapy implicated in hypomagnesaemia (e.g., proton pump inhibitors, antibiotics, etc.,), any overt causes of pre-term birth (bacterial vaginosis, cervical incompetence, premature rupture of membranes, etc.,), known cases of Gestational Diabetes Mellitus (GDM), DM, Pregnancy Induced Hypertension (PIH) or primary hypertension, hypothyroidism or hyperthyroidism and assisted deliveries or Caesarean sections were excluded from the study.
Statistical Analysis
Statistical analysis of data was done using IBM SPSS (Statistics Package for the Social Sciences) Statistics for Windows, Version 21.0. (Armonk, NY: IBM Corp) and Microsoft Excel 2016. All continuous variables were expressed as mean±standard deviation and all categorical variables as a percentage. The normality of continuous data was assessed by the Shapiro-Wilk test. Pearson’s correlation analysis was used to determine the association between continuous variables. Comparison of means of different groups was done using a t-test. A p-value< 0.05 was considered statistically significant.
Results
The study consisted of pregnant women (n=110) aged between 18 to 36 years (Mean=26.8, SD=3.25), admitted for delivery. A total of 44 out of 110 participants (40.0%) had serum Mg levels ≤1.8 mg/dL and were hypomagnesaemic. Mean maternal magnesium level was 1.93±0.31 mg/dL. Mean cord blood magnesium level was 2.19±0.28 mg/dL. Total 58 out of 110 participants (52.73%) were vegetarians, and the remaining 52 out of 110 were non-vegetarians (47.27%). Among 110 women, 41 were primigravida/nullipara (37.20%).
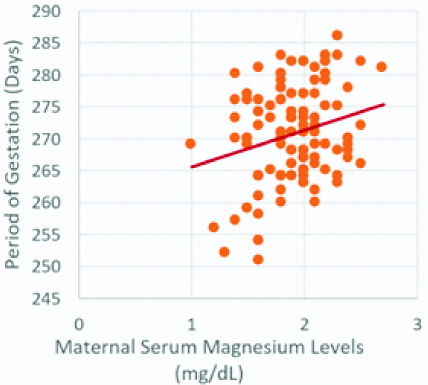
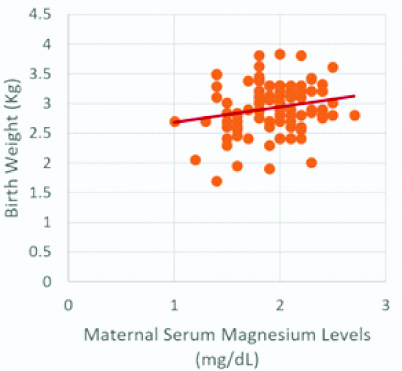
Maternal serum Mg levels were positively correlated with the period of gestation (r-value=0.246, p-value <0.01), which was statistically significant [Table/Fig-1,2]. Similarly, maternal serum Mg levels were positively correlated with birth weight (r-value=0.205, p-value <0.05), which was statistically significant [Table/Fig-1,3].
Correlation coefficients for period of gestation, birth weight and maternal and cord blood magnesium levels.
| | Period of gestation | Birth weight |
|---|
| Maternal magnesium levels | Pearson’s correlation r | 0.246** | 0.205 |
| Significance (Two-tailed) p | 0.0090 | 0.0314 |
| N | 110 | 110 |
| Cord blood magnesium levels | Pearson’s correlation r | -0.240 | -0.303 |
| Significance (Two-tailed) p | .0113 | 0.0012 |
| N | 110 | 110 |
*p<0.05 statistically significant; **p<0.01 statistically highly significant
Scatterplot for period of gestation and maternal serum magnesium levels.
Period of gestation and maternal serum magnesium levels show a significant positive correlation (r-value=0.246, p-value <0.01)
Scatterplot for birth weight and maternal serum magnesium levels.
Birth weight and maternal serum magnesium levels show a significant positive correlation (r-value=0.205, p-value <0.05)
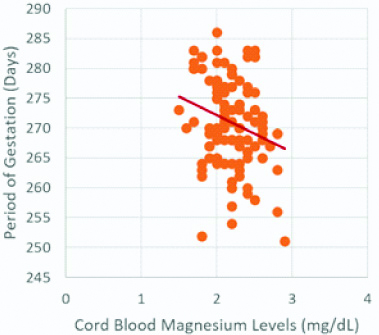
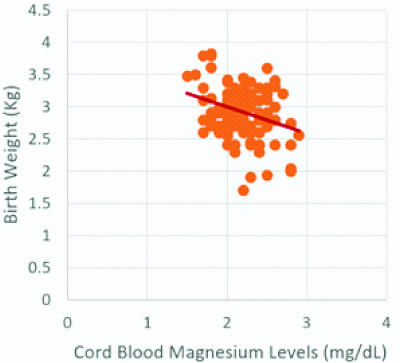
A Pearson correlation coefficient was computed to assess the relationship of cord blood Mg levels with period of gestation and birth weight [Table/Fig-1]. Statistically significant negative correlation between cord blood Mg levels and period of gestation (r-value=-0.240, p-value <0.05), and cord blood Mg levels and birth weight (r-value=-0.303, p-value <0.01) was observed [Table/Fig-1,4,5].
Scatterplot for period of gestation and cord blood magnesium levels.
Period of gestation and cord blood magnesium levels show a significant negative correlation (r-value =-0.240, p-value <0.05)
Scatterplot for birth weight and cord blood magnesium levels.
Birth weight and cord blood magnesium levels show a significant negative correlation (r-value=-0.303, p-value <0.01)
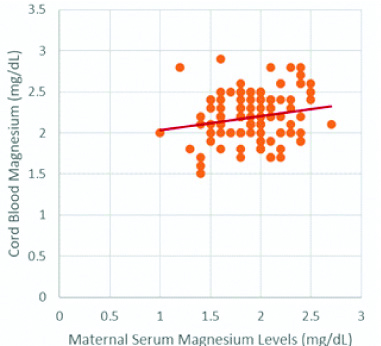
No significant correlation was observed between maternal Mg levels with maternal age, gravidity, or parity. Concurrently there was no significant correlation of cord blood Mg levels with these variables. However, maternal and cord blood magnesium levels showed a weak positive correlation (r-value=0.188, p-value=0.048) [Table/Fig-6].
Scatterplot for maternal serum magnesium and cord blood magnesium levels.
Maternal serum magnesium and cord blood magnesium levels show a significant positive correlation (r-value=0.188, p-value <0.05)
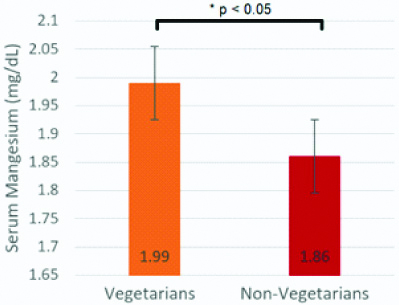
Amongst vegetarians, 25.86% (15/58) were hypomagnesaemic, and amongst non-vegetarians, 55.77% (29/52) of participants were hypomagnesaemic. An unpaired t-test was conducted to compare maternal serum Mg levels in vegetarian and non-vegetarian groups. On average, the maternal serum Mg levels in vegetarians were higher (1.99±0.30) than the maternal serum Mg levels in non-vegetarians (1.86±0.32), and the difference was statistically significant (t-value=2.14, p-value=0.034) [Table/Fig-7].
Maternal serum magnesium levels (Mean±SD) in vegetarian and non-vegetarian subjects.
Mean maternal magnesium levels were significantly greater in vegetarians than in non-vegetarians (t-value=2.14, p-value <0.05)
Discussion
Mg, unlike some other minerals, has not only been overlooked in its importance in being responsible for a plethora of disease states, but there is also a lack of any substantial literature on the same. Only scattered literature exists on the prevalence of hypomagnesaemia in the general population. Pregnant women are particularly vulnerable to nutritional deficiencies, especially in developing countries, with Mg being no exception. Moreover, hypomagnesaemia has been postulated to be associated with pre-term labour and IUGR too. This study was taken up to assess the prevalence of hypomagnesaemia in an urban setting, and by simultaneously measuring the cord blood levels of Mg, other parameters were also correlated.
Pathak P et al., studied the prevalence of multiple micronutrient deficiencies amongst pregnant women in rural Haryana reported the prevalence of Mg deficiency to be 43.6%. The study had enrolled pregnant women of >28 weeks period of gestation, from six villages [14].
The prevalence of hypomagnesaemia amongst pregnant women in our setting was 40.0%, similar to that obtained in the above-mentioned study. Interestingly, though significant differences in diet and nutritional status were expected to exist between pregnant women residing in rural and urban areas, owing to differences in the availability of food products, socioeconomic status, dietary habits, and local knowledge, attitudes, and practices, we found a similar prevalence of hypomagnesaemia. Our study was conducted in a large urban tertiary care center. While inadequate dietary intakes are expected to be more common in a rural population, the prevalence of Mg deficiency in our study points towards more factors contributing to Mg deficiency, other than rural and urban settings.
A high-calorie intake does not necessarily correlate with an adequate intake of micronutrients. As dietary trends in the urban areas of India lean towards increased intake of processed foods, the micronutrient densities of food are chronically low. The western diet has been shown to be Mg deficient [9].
In our study, 52.73% (58/110) of participants were vegetarians, and the remaining 47.27% (52/110) were non-vegetarians. Amongst vegetarians only 25.86% (15/58) were hypomagnesaemic, and amongst non-vegetarians, 55.77% (29/52) of participants were hypomagnesaemic. It shows that the non-vegetarian diet is rich in protein, heme iron, essential fatty acids, and vitamin B, may not amount to an adequate intake of Mg or other micronutrients. A balanced diet, rich in food groups including vegetables, fruits, and nuts during pregnancy as essential for maintaining an optimum Mg balance.
The increased prevalence of hypomagnesaemia in our urban setting is perhaps attributable to a reduced intake of Mg-rich food groups -vegetables, nuts, fruits, and oilseeds-primarily due to lifestyle choices. As processed foods continue to dominate the Indian market, micronutrient deficiencies will tend to ensue.
Maternal serum Mg levels were found to be positively correlated with the period of gestation and birth weight in our study. A short period of gestation, therefore, points towards Mg deficiency, and our findings were similar to those reported in other studies [13,15]. Mg ions play a significant role in the stabilisation of cellular membranes, and their actions are opposite to that of calcium ions at the molecular level on muscle contraction and nerve impulse conduction. Mg promotes the re-uptake of calcium ions in the sarcoplasmic reticulum of muscle cells, by the calcium-activated ATPase. This leads to a reduced concentration of calcium in the muscle cells. Mg deficiency possibly leads to a low concentration of Mg in the myometrium of the uterus, leading to increased uterine contractility and pre-term labour. Mg has been suggested to act on the uterus in a dose-dependent manner, and the inhibitory effect on uterine contractions varies with oxytocin levels [16]. An adequate dietary intake of Mg throughout the duration of pregnancy may, therefore, prove to be a holistic approach to the prevention of pre-term births.
Pre-term birth is directly related to infant mortality, low birth weight and other complications at birth. Hypomagnesaemia may be a potential risk factor for pre-term labor. Total serum Mg estimation is a simple, rapid and very cost-effective tool and antenatal screening to identify high-risk cases may prove beneficial.
We found that cord blood Mg levels were significantly correlated with both the period of gestation and the birth weight of the infant. The correlation was negative, with an increasing Mg level of the infant associated with shorter periods of gestation and lower birth weights. A short period of gestation allows for less time for foetal development in-utero and is closely linked with low birth weight and complications that may occur later on at birth. Birth weight of the newborn is a powerful predictor for a wide range of undesirable outcomes and is the most important factor that affects neonatal morbidity and mortality. Low birth weight infants are at increased risk of respiratory distress, hypotonia, infection, and chronic diseases later in life.
On comparing other parameters we found no significant correlations between maternal Mg levels and gravidity or parity or maternal age. Socioeconomic factors in rural areas, such as attitudes toward family planning and the availability and popularity of contraceptives are important aspects of public health. In our setting, the mean gravidity and parity were relatively low. Mg deficiency might be more predominant in grand multiparous women with smaller gaps between pregnancies, prevalent in rural areas across India, not relevant to our study.
Limitation and Future Prospects
The current study establishes an association between maternal and cord blood Mg levels with period of gestation and birth weight. However, the role of Mg in the causation of low birth weight or low period of gestation cannot be identified with cross-sectional data. Furthermore, the use of food frequency questionnaires or 24-hour dietary recall would better demonstrate the effect of dietary factors on Mg status. This would require extensive follow-up and recruitment of pregnant women from earlier trimesters of pregnancy, which was not possible in this study. Hence, a prospective study design with a larger sample size especially amongst high-risk cases for preterm labour in India will be useful to assess the benefits of a Mg-rich diet during pregnancy or Mg supplementation.
Conclusion
Hypomagnesaemia was highly prevalent amongst pregnant women in our setting. An inadequate intake of Mg-rich food could be a possible cause. Mg is associated with a number of deficiency disorders including problems specific to pregnancy. We demonstrated a significant correlation between Mg deficiency and shorter periods of gestation and low birth weight. Cord blood Mg levels were found to be inversely correlated with period of gestation and birth weight, both of which are predictors for adverse neonatal outcomes. Maternal age, gravidity, and parity did not have any effect on Mg levels in our study. In conclusion, larger, trimester-specific studies for antenatal screening to elucidate Mg deficiency, and alongside an all-round patient educational approach emphasising Mg-rich diet or Mg supplementations as the need may be, could be highly beneficial to pregnant women in India.
*p<0.05 statistically significant; **p<0.01 statistically highly significant