Urinary bladder cancer is the ninth most common cancer worldwide. The most frequent histologic subtype (>90%) is Urothelial carcinoma [1]. It is more common in males (male: female ratio- 3:1) [2]. The gross appearance of urothelial carcinoma may be exophytic or endophytic. Microscopically, the invasion to sub epithelial tissues proceeds in two stages: (a) invasion of the lamina propria which is difficult to detect and (b) invasion of the muscle layer, which has great significance because of its influence on therapy and prognosis.
Urologists over decades have accepted the limitations of radiological methods for detecting urothelial carcinomas, more so for flat urothelial lesions [3]. However, cytological examination of urinary sediment is now accepted as a part of routine investigation of patients presenting with haematuria, prostatism, or others suggesting urothelial neoplasia [4]. Urine cytology can detect high grade carcinomas as well as carcinoma in-situ, the latter being flat lesion is difficult to diagnose cystoscopically [5].
Despite its relevance as a diagnostic tool, urine cytology has faced great controversy, mostly due to its inherent inability to detect low grade neoplasms. Establishing exact correlations in nomenclature between histological and cytologic findings were also difficult in the past. It also suffered from wide range of sensitivity depending on the grade of the tumour and the questionable standards of specimen collection, preservation, processing. Hence, for practical use, the need for a concise yet specific system to report urine cytology was felt. The main goal was efficient detection of clinically significant urothelial carcinoma, namely HGUC [6]. In late 2015, The Paris System (TPS) for Reporting Urinary Cytology was published which promised an adequately specific system for reporting urine cytology [7].
The p53 is one of the principal antigens, which have been well studied and documented in the immunohistology of urinary bladder [8]. It is a tumour suppressor gene that transcriptionally regulates control on cell cycle [9]. Studies have revealed p53 mutations in 40% to 60% of invasive bladder cancers and established their association with high grade urothelial neoplasm and advanced stage of the disease [10-15]. Owing to the extended half-life of the altered protein product of mutant p53 gene, its accumulation and detection is well experimented and proved by immunohistochemical techniques [16]. The p53 alterations in urothelial carcinomas have also been shown to be predictive of increased sensitivity to chemotherapeutic agents that damage DNA [17,18].
The aim of this study was to evaluate the diagnostic accuracy of urine cytology as per TPS correlating it with the histopathological subtypes and immunohistochemical expression of p53 in urothelial neoplasms and to access the utility of TPS at our resource limited government run institution. Herein we report our experience using this new classification for 18 months.
Materials and Methods
It was a descriptive cross sectional study using systematic random sampling design. The study consist size of 75 samples, studied over a period of 18 Months (January 2017-June 2018).
Cases included patients who were clinico-radiologically diagnosed with urothelial neoplasms in the urinary bladder. Excluded categories comprised urothelial carcinomas at other sites like renal pelvis and ureter, mesenchymal tumours, primary adenocarcinomas, pure squamous cell neoplasms and inflammatory conditions of bladder.
Informed written consent was obtained from all the subjects. Medical records, relevant history and clinical data were collected including age, sex, religion, occupation, addiction, dietary factors, literacy, comorbidities, clinical presentation and radiological findings. The dependent variables in this study included urine cytology (reported as per TPS), histopathology and immunohistochemical analysis of expression of p53 mutation.
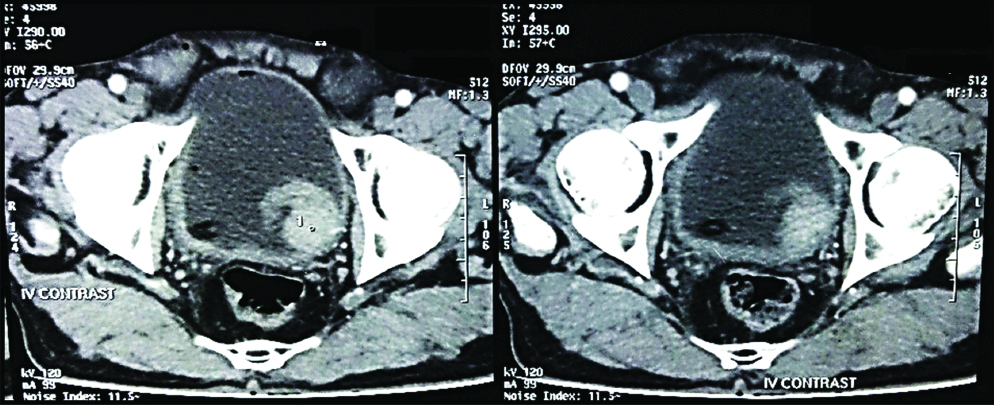
After approval from the Institutional Ethical Committee (Ethical Clearance number- RMC/4969) the study was initiated. The cases were collected from both the Outdoor and Inpatient Department of Urosurgery. The subjects that presented clinically with symptoms suspicious of urothelial carcinomas like painless, gross haematuria, nocturia, dysuria or urgency of micturition and were diagnosed radiologically (ultrasonography/ CT scan/MRI) with urothelial neoplastic lesions were followed [Table/Fig-1,2].
Contrast enhanced CT scan, showing a Space Occupying Lesion (SOL) in the Left postero-lateral wall of Urinary Bladder.
Cystoscopic appearance in a case of Urinary Bladder SOL, prior to Trans-Urethral Resection Of Bladder Tumour (TURBT).
For cytological examination, [19] the early morning mid-stream voided urine samples were collected in a clean glass tube. The samples were centrifuged within 3-5 hours in a graduated Borosilicate glass centrifuge tubes at a speed of 3000 rpm for 10 minutes in Remi’s centrifuge machine. The supernatant was poured out and smears from urinary sediment were made on clean, grease free glass slides. The smears were fixed in 95% Alcohol for 2 hours, stained with Papanicolaou stain and examined under light microscope for malignant cells. The slides were screened by four pathologists of the same department. The inter and intra observer variability was insignificant. The cases were assessed as per the criteria stated under TPS and appropriate diagnostic grade were assigned which include:
Non diagnostic or unsatisfactory,
Negative for High Grade Urothelial Carcinoma (NHGUC),
Atypical Urothelial Cells (AUC),
Suspicious for High Grade Urothelial Carcinoma (SHGUC),
High Grade Urothelial Carcinoma (HGUC),
Low Grade Urothelial Neoplasms (LGUN).
The surgical specimen received was processed and stained with Haematoxylin and Eosin (H&E) and histopathologically evaluated for diagnosis, histological type and grading of Urothelial Carcinoma according to WHO (2016) and AJCC cancer staging (2017).
Immunohistochemical analysis was performed using commercially available mouse monoclonal antibodies for p53 expression in paraffin embedded sections in all cases [20]. The sections taken from the specimen were kept overnight in 10% neutral buffered formalin and thereafter standard steps of paraffin block preparation were initiated. With the help of microtome (Leica-RM2125 RT), ribbon-like sections of 4μ thickness, were cut and floated in warm water (~56°C). These sections were then collected on poly-L-lysine coated slides. After deparaffinization and washes in wash buffer, antigen retrieval was done by Microwave method. It was followed by endogenous peroxide-blocking using 3% hydrogen peroxide for 5 minutes at room temperature). Then primary antibody was added (Clone-CELL MARQUE D 07), followed by amplifier and HRP label. Horseradish peroxidase (HRP) is the enzyme which in presence of H2O2 and a chromogen (3,3/Diaminobenzidine tetrahydrochloride-DAB), identified the site of antibody binding by forming a crisp, insoluble, stable, dark brown coloured reaction end product). It was subsequently followed by DAB visualisation and Haematoxylin counterstaining. Cases of Invasive Breast Carcinoma, staining positive for p53 were taken as positive control. Normal urinary bladder epithelium served as the negative controls.
Cytology as per TPS was arranged as NHGUC, AUC [Table/Fig-3], SHGUC [Table/Fig-4], HGUC [Table/Fig-5]. Histopathology categories were Inverted Papilloma [Table/Fig-6], Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP) [Table/Fig-7], Low Grade Papillary Urothelial Carcinoma (LGPUC) [Table/Fig-8], High Grade Papillary Urothelial Carcinoma (HGPUC), and Invasive Urothelial Carcinoma (invasive) [Table/Fig-9]. Interpretation of p53 expression- Brown coloured nuclear staining. For reporting, the following scoring protocol was followed: <10%- negative [Table/Fig-10], 10-49%-weakly positive [Table/Fig-11], ≥50%-strongly positive [Table/Fig-12] [15]. As per recommended statistical guidelines for qualitative data, the test diagnostic accuracy was calculated using specific diagnostic thresholds.
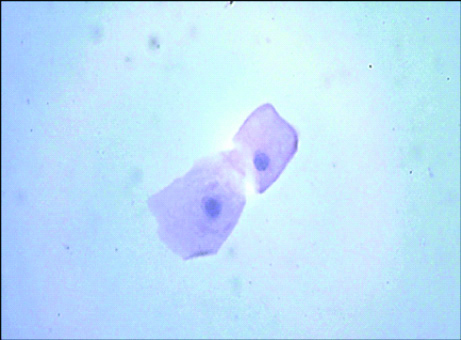
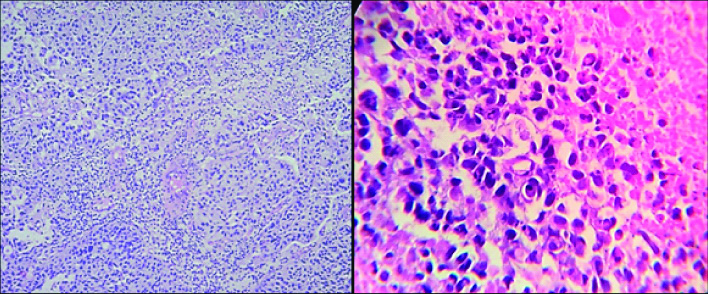
Photomicrograph of ATYPICAL UROTHELIAL CELLS in cytology as per TPS (Papanicolaou; 400 X view).
Photomicrograph of SHGUC in urine cytology as per TPS (Papanicolaou; 400 X view).
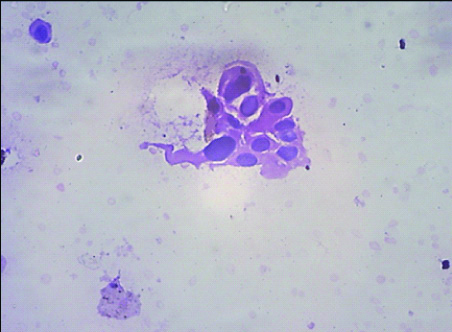
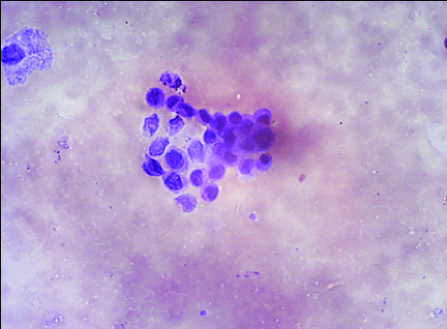
Photomicrograph of HGUC in cytology as per TPS (Papanicolaou; 400 X view).
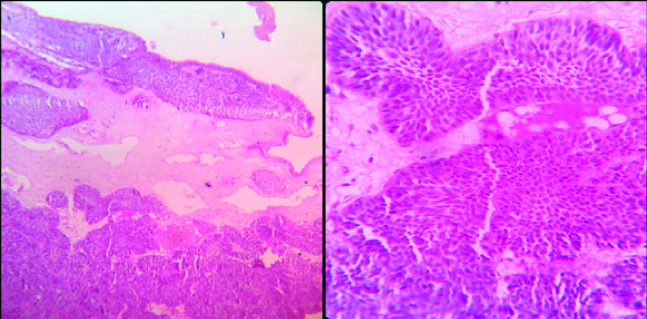
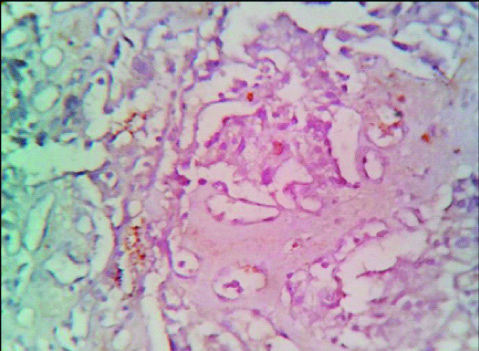
Photomicrograph of Histopathology of Inverted Papilloma (Left- H&E; 40 X, right- H&E; 100 X).
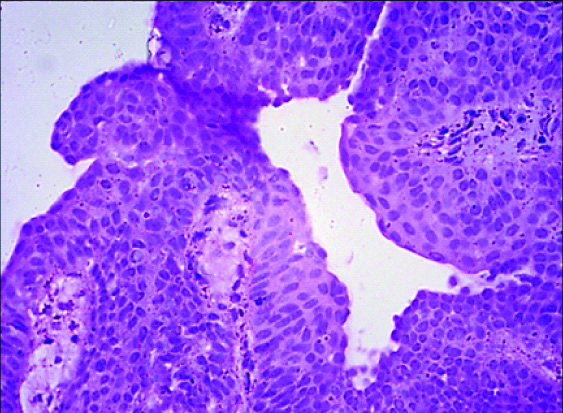
Photomicrograph of Histopathology of Papillary Urothelial Neoplasm of Low Malignant Potential (H&E; 400 X).
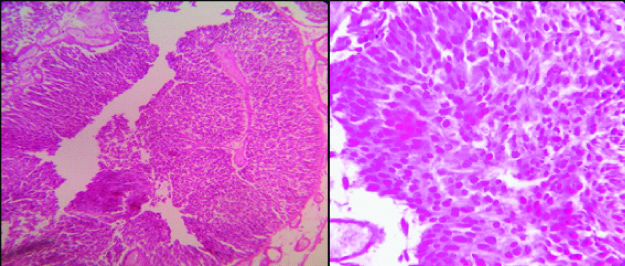
Photomicrograph of Histopathology of Low Grade Papillary Urothelial Carcinoma (Left- H&E; 100 X, right- H&E; 400 X).
Photomicrograph of Histopathology of Invasive Urothelial carcinoma (Left- H&E; 100 X, right- H&E; 400 X).
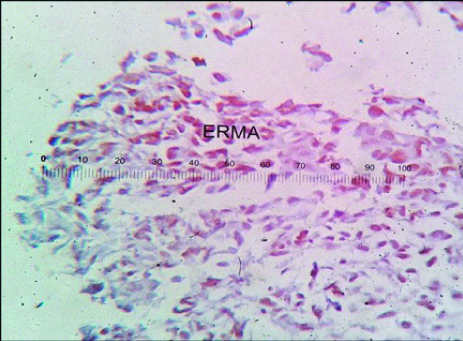
Photomicrograph of p53 negative expression in a case of Low grade papillary Carcinoma (100X).
Photomicrograph of Weak p53 positivity in a case of Invasive Urothelial Carcinoma (400 X).
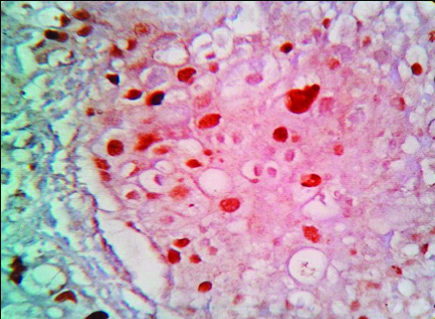
Photomicrograph of Strong p53 positivity in Invasive Urothelial Carcinoma (100 X).
Statistical Analysis
Microsoft Excel 2016 was used for data entry and the data analysis was done using Statistical Package for Social Science (SPSS version 25.0). The diagnostic categories for each tests were noted and respective frequencies were obtained. The mean age was calculated to be 60 years. The most common site in bladder was found to be the right lateral wall. The male to female ratio was observed to be 9.7 to 1.
For correlation between the three tests, histopathology was considered as the gold standard test. The diagnostic categories in each tests arranged in the order of lower to higher grade, served as the variables.
Pearson’s chi-squared tests were performed for correlation of qualitative data and determine whether there is a significant difference between the expected and observed frequencies in one or more categories. The association between two variables is considered statistically significant if Asymptotic Significance (2-sided) is <0.05 (p-value).
Results
Of 75 cases, 65.3% cases (49/75) showed cells eliciting histopathological features of high grade carcinoma [Table/Fig-13]. Urine cytology as reported by TPS showed 69.4% cases having high grade nuclear features, of which, 38.7% were designated as high grade urothelial carcinoma due to greater cellular yield of such cells [Table/Fig-14]. An amount of 74.7% of cases (56/75) stained positively for p53, indicating the presence of high grade cells in the lesion [Table/Fig-15].
Frequencies of histopathological diagnoses.
| Histopathology | Frequency | Percentage |
|---|
| Inverted papilloma | 2 | 2.70% |
| PUNLMP | 9 | 12% |
| LGPUC | 15 | 20% |
| HGPUC | 4 | 5.30% |
| Invasive carcinoma | 45 | 60% |
| Total | 75 | 100% |
Frequencies of cytological diagnoses as per the paris system.
| Cytology | Frequency | Percentage |
|---|
| NHGUC | 11 | 14.60% |
| AUC | 12 | 16% |
| SHGUC | 23 | 30.70% |
| HGUC | 29 | 38.70% |
| Total | 75 | 100.00% |
Frequencies of different grades of p53 immunostaining.
| Grades of p53 | Intensity | Frequency | Percentages |
|---|
| Negative | <10% | 19 | 25.30% |
| Weak positive | 10-49% | 29 | 38.70% |
| Strong positive | ≥50% | 27 | 36% |
| Total | | 75 | 100% |
The distribution of the cases, taking SHGUC as diagnostic threshold of urine cytology, correlated with the histopathology is depicted in [Table/Fig-16]. The table shows eight cases which were falsely positive for high grade in cytology attributable to poor preservation of cells after processing of urine sample leading to morphological distortion and diagnostic dilemma.
3D area diagram showing the cytological and histopathological diagnoses using SHGUC as threshold in cytology.
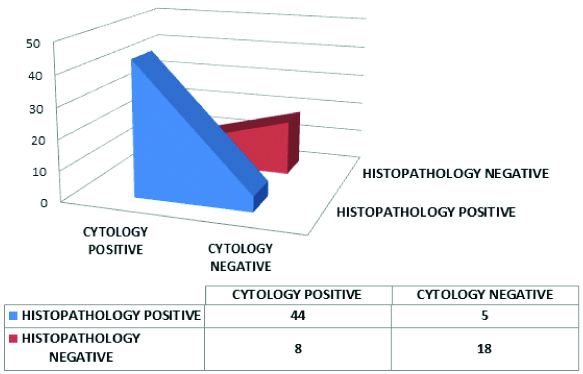
The sensitivity and specificity of urine cytology was calculated to be 89.80% and 69.23% respectively. The positive predictive value was 84.62% and negative predictive value was 78.26%.
The likelihood ratio for a positive report i.e. LR(+) on cytology was computed to be 2.92 (>1), indicating that cytology when reported as per TPS, taking SHGUC as cut-off for positivity, was more likely to give a positive test result in patients with high grade lesion on histopathology than in patients with low grade lesions. The likelihood ratio for a negative result in cytology LR (-) was computed to be 0.15. This value indicates a strong association between having a negative cytology result and having a low grade lesion.
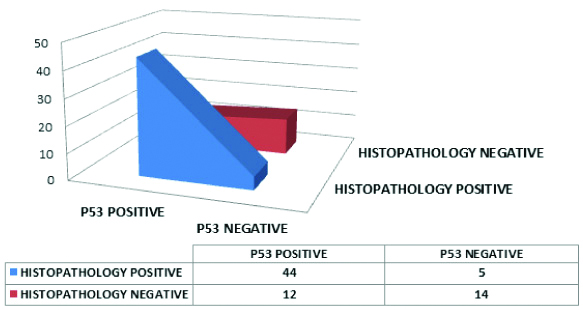
Correlation of p53 with histopathology taking 10% as the cut-off for positivity is depicted in [Table/Fig-17]. The table shows 12 cases which were falsely positive for p53 attributable to the false overexpression of p53 in cases which were not high grade in histopathology. The statistical analysis done by chi-square test revealed that the p-value is <0.05. The correlation of the results of p53 and histopathology when 50% was used as the cut-off was shown in [Table/Fig-18]. The test yielded a low sensitivity of 53.06% and specificity of 96.15%.
A 3D area diagram showing the p53 and histopathological diagnoses using p53 weak as threshold for positive for p53 immunostain
The 2x2 contingency table for correlation between p53 and histopathology after using p53 strongly positive as diagnostic thresholds in p53.
| HP status | p53 positive | p53 negative | Total |
|---|
| HP positive | 26 | 23 | 49 |
| HP negative | 1 | 25 | 26 |
| Total | 27 | 48 | 75 |
Discussion
Urine is a heterogeneous mixture, containing non solute particles, crystals, microorganisms as well as cells shed from the entire urinary tract along with chemical substances like urea, creatinine, that markedly affect the osmolality of urine. Hence, preservation and distribution of cells throughout the urine volume is not uniform. As per TPS, a urine sample could be called inadequate only if it did not show any finding indicative of any disease process or if urothelial cells are obscured by lubricants, mucin or blood.
TPS has clearly achieved its goal of reducing the rate of “atypical” cells in urine cytology reporting since all reactive changes are included in the category of “negative” urine cytology. Hence, whereas previously, subjects assigned with a diagnosis of “atypical” cells were managed in a way similar to “negative” cases, after the implementation of TPS, the criteria of AUC group becoming clearer, such cases are managed by closer follow-up and evaluation. TPS also cleared the distinction between SHGUC and HGUC. While cellular morphological criteria for both the groups were similar, the difference between them was clearly stated to be the quantity of cells showing such features.
There is a significant association between cytology and histopathology in diagnosing high-grade urothelial carcinoma cases (p<0.05) as observed in this study. Significant association was also noted between p53 and histopathology in diagnosing high grade urothelial carcinoma cases (p=0.001).
The parameters of diagnostic accuracy with different cut-off for cytology were compared with previous studies and tabulated in [Table/Fig-19,20]. These were found to be concordant with studies by Hassan M et al., and Roy M et al., but non concordant with Rohilla M et al., presumably due to restricted sample collection in this study [21-23].
Table comparing the statistical figures of the present study with previous studies.
| Studies | Sample size | Male: female ratio | Median age (years) |
|---|
| This study | 75 | 9.7 to 1 | |
| Hassan M et al., [21] | 124 | 2.4 to 1 | 73.8 |
| Roy M et al., [22] | 97 | 9.8 to 1 | 56.7 |
| Rohilla M et al., [23] | 1396 | 3.7 to 1 | 54 |
| Chan E et al., [24] | 188 | 3 to 1 | |
Table comparing the statistical parameters of the present study that of previous studies, when SHGUC is taken as the threshold for positivity in cytology.
| Variables | This study | Hassan M et al., [21] | Roy M et al., [22] | Rohilla M et al., [23] | Chan E et al., [24] |
|---|
| Positive predictive value | 84.6% | 93.6% | 89.5% | 94.8% | 90.3% |
| Negative predictive value | 78.30% | 67.5% | 70% | 32.2% | 88.9% |
| Sensitivity | 89.8% | 63.7% | 80.9% | 70.5% | 62.2% |
| Specificity | 69.2% | 94.5% | 82.4% | 78.4% | 97.8% |
| Positive likelihood ratio | 2.92 | 11.7 | 4.59 | 3.26 | 28.8 |
| Negative likelihood ratio | 0.15 | 0.38 | 0.23 | 0.38 | 0.39 |
| Diagnostic accuracy | 82.67 | 77.4 | 81.4 | 71.7 | 89.1 |
Taking SHGUC as cut-offs for positive in cytology, the study was found to be in accordance with studies of Chan E et al., [24] and Roy M et al., [22] respectively hence SHGUC can be considered a better cut-off for positive cytology.
For correlation between p53 and histopathology, the first cut-off chosen for positivity in immunochemical examination is p53 weak positive (10-49%) which was in accordance to a study by Salinas Sánchez AS et al., and Erill N et al., [25,26]. Hence week positivity of p53 can be chosen as a cut-off for immunohistochemistry.
ROC curve was prepared for visually describing the intrinsic accuracy of the test [Table/Fig-21]. The chance diagonal line was drawn joining coordinates (0, 0) and (1,1). The black line joining coordinates (0,0) and (1,1) is known as the chance diagonal. The coordinate (0,1) is known as point ‘C’. as per statistical measures, the diagnostic point in the test graph which is farthest from the chance diagonal and closest to point ‘C’ is considered as the best diagnostic threshold. Hence, from the graph of cytology (Blue), the best diagnostic cut-off for positivity is SHGUC. This was in accordance with study by Fite JJ et al., [27].
ROC curve for qualitative data plotted on a graph with (1-specificity) on X-axis and sensitivity on Y-axis.
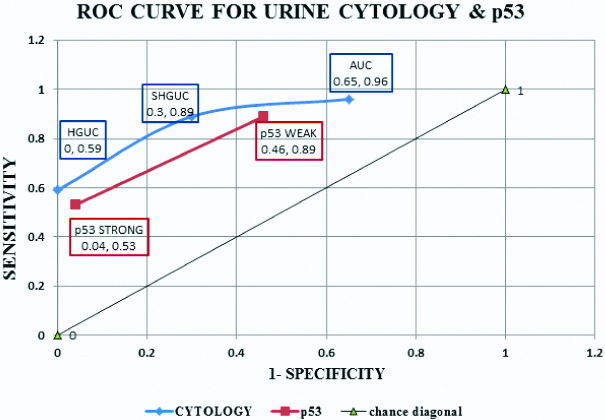
In this study, p53 immunohistochemical marker was correlated with the high grade cases on histopathology. This indicated a greater association of p53 status with a poor prognosis and increased rates of progression to invasive forms in urothelial carcinomas.
Limitation
This study have few limitations. Urine cytology could not specifically diagnose the low grade neoplasms and were reported as the absence of high grade tumour cells. Overall specificity of urine cytology was less, which indicated false reporting of atypical cells in cytology, attributable to the poor preservation of cell morphology after specimen collection. The increased number of false positives in p53 staining could be attributed to artefactual deposition of stains in the tissue sections. The study was also limited by smaller sample size, hospital study bias and minimal infrastructural facilities.
Conclusion
Overall, significant association was found between p53 positive cases and cases diagnosed as SHGUC and HGUC in cytology as reported by TPS with cases that showed presence of high grade cells on histopathological examination.
Future Recommendations
Awareness is needed among the treating physicians regarding the ease of using urine cytology as a test modality to approach patients presenting with symptoms suggestive of urothelial carcinoma, especially among low socio economic groups. With the advent of TPS, there are clear cut guidelines making treatment simpler. Patients should also be followed-up more closely and offered active interventions to eradicate invasive and recurrent tumours.