The human oral cavity is densely populated with various microorganisms, including bacteria, viruses and fungi [1]; these microorganisms play the significant roles in health and can cause oral diseases [2].
Natural products offer pharmacological benefits either by direct use as treatment or as bioactive compounds in the development of drugs [5]. Soursop (Annona muricata L.) leaves have various anti-inflammatory, antihypertensive, anticancer, and antimicrobial properties. Their chemical constituents are flavonoids [6], polyphenols, and proteins, which have confirmed antimicrobial properties against bacteria such as Staphylococcus aureus and Pseudomonasaeruginosa [7].
Because soursop is abundant in Peru, it is of great agricultural and industrial interest [8].
The literature currently has inadequate evidence on the use of Soursop leaf extract (Annona muricata) on systemic pathogens, oral pathogens [9-16]. Hence, the present study is an archetypal report of the anti-microbial potential of Soursop on a common oral pathogen using an in-vitro study model, with the objective to compare the antibacterial effects of different concentrations of A. muricata L. leaf extracts on S. mutans ATCC 25175 strains.
Materials and Methods
This study used an in-vitro experimental design in a laboratory setting, carried out during May 2017 to August 2018. Microbiological observation and the inhibition zones were measured using a millimeter ruler (DIN/ISO 866/I).
Soursop leaves were collected from a plot in Bagua Grande in the Amazon and taken to the herbarium of the National University of Trujillo (Truxillense Herbarium), where the taxonomic classification of the A. muricata was determined, with code N° 59287.
Five kg leaves were collected from trees in Guanabo, Bagua Grande, in May 2017 and placed in a cardboard box with holes. The box was taken to the School of Pharmacy and Biochemistry of the National University of Trujillo for further processing [Table/Fig-1].

Preparing the Ethanolic Extract from A. muricata L.
The leaves were collected in the morning, when possible using the conventional method, and the leaves were verified to be in good condition [Table/Fig-1]. The leaves to be used were selected; damaged and withered leaves were removed. Fresh samples were rinsed with distilled water and dried in open air. The leaves were dried to avoid alterations that could affect their composition. The selected leaves were placed on kraft paper in a cool and dry place for 24 hours, transferred to trays made of kraft paper and placed in a stove at 40°C for 48 hours [Table/Fig-1].
After drying, the samples were grounded and homogenised in an industrial mill and then sieved (to a 2-mm particle size) [17-19].
Extract Preparation by Leaching
The ethanolic extracts were prepared by leaching, as described in the United States Pharmacopoeia, USP. The leachate crosses the powdered drug in only one direction, achieving increasing concentrations, so that the solvents inside and outside the stage never reached an equilibrium. New leachate always flows through the drug, which progressively confers its soluble constituents [20]. One hundred grams of the powdered drug was weighed, placed in a large container, moistened with 200 mL of 96°C alcohol, and then left for 1 hour.
✓ A cotton swab was placed in the percolator’s outlet and then the moistened drug was transferred. A filter paper plate was placed on the leachate surface and pressed.
✓ No air bubbles were allowed to form in the plant material. The alcohol was added with the outlet of the leach reactor open; the leachate then flowed, and the exit was quickly shut. The alcohol was then poured until it completely covered the plant material to a level of 3 to 5 cm above the mass, which was macerated for 24 hours.
✓ After 24 hours, 75 mL of the extract was directly obtained and transferred to an amber bottle.
✓ The exit outlet was opened, and more alcohol was added until a flow rate of 25-30 drops/min was established to obtain the filtrate of the second portion, which was a lighter liquid; a volume of 400 mL was obtained.
✓ The collected 400 mL extract was concentrated at 50°C at 200 rev/min, finally obtaining 70 mL by reduction.
✓ The first extract collected was added to the second.
✓ One hundred milliliters of alcoholic extract was obtained from 100 mL of crude drug, which was conserved in an amber glass bottle.
✓ The extract was refrigerated and stabilised at 6°C to 8°C [Table/Fig-1] [21,24].
Measuring Concentrations
The liquid soursop leaf extract was filtered in a vacuum pump with Whatman No. 1 filter paper. The extract was then evaporated on a rotary evaporator at 40°C until a syrupy mass was obtained, which was subsequently dried in an oven at 40°C until the dry extract was obtained. From the dry extract, 10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80% concentrations were prepared by dissolution in 70% ethanol. Finally, the ethanolic extracts from each concentration were stored in amber glass bottles and refrigerated (4-8°C) until use [Table/Fig-1].
Activation of S. mutans
✓ A commercial bacterial strain of S. mutans (ATCC 25175) was provided by the Laboratory of the National University of Trujillo.
✓ The vial was opened as per the instructions, and the bacterial strain was reconstituted with 3 mL of liquid BHI.
✓ Seeding was performed in four tubes, each containing 10 mL of BHI, and 0.5 mL of BHI medium containing the strain was added. The tubes were then placed in a jar and kept in a stove at 37°C for 24 hours.
✓ The strain was standardised per the McFarland 0.5 scale using an S. mutans inoculum in 2 mL of BHI broth until it obtained the same turbidity as the McFarland 0.5 standard, representing a known microorganismal concentration of 1.5×108 Colony-Forming Units (CFU) [3].
Antibacterial Activity
The inoculum was prepared to an optical density of 0.688 and seeded. [The optical density of the S. mutans suspension was measured as 0.012. The optical density of the medium (0.700) was used as the control. The suspension was prepared by adding 5454 μL of BHI with S. mutans and 549 μL of liquid BHI suspension.
Twenty-one 100-mm plates were prepared with BHI agar medium, and 20 mL of previously autoclaved medium was placed into each plate and sterilised using a lighter.
The medium was cooled for one hour.
The inoculum was seeded on the plates using a loop and swab in three stages, and the plate was turned to homogenise the seeding [25-27].
The 6-mm-diameter wells were then prepared, and the extracts were added in order of concentration and labeled 1 to 8.
Seventy percent ethanol was the negative control, and 0.12% chlorhexidine gluconate was the positive control.
The extract was absorbed for few minutes, and then the plates were coated with paraffin and placed in anaerobic jars. The jars were placed in the stove at 37°C for 48 hours and then removed [28-31]. Readings were taken by measuring the diameter of the inhibition halos [Table/Fig-2] [32,33].
Statistical Analysis
SPSS software was used for statistical analysis (version 23.0). The diameters of the inhibition values of all different concentrations were entered in the SPSS software for statistical analysis. Descriptive statistics were retrieved and data was analysed using one way analysis of variance (ANOVA) and Duncan test. Statistical significance level was established at p<0.05.
Results
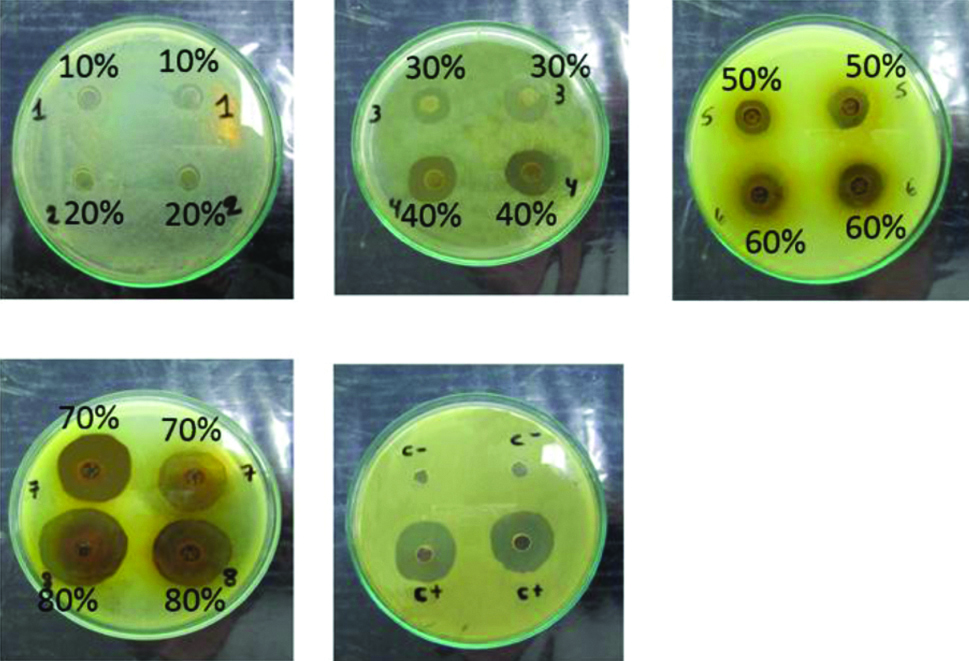
[Table/Fig-3] shows that the antibacterial effects on the S. mutans ATCC 25175 strains differed significantly (p<0.05) between the concentrations of ethanolic A. muricata L. leaf extracts.
In-vitro comparison of the antibacterial effects of different concentrations of ethanolic A. muricata L. leaf extracts on Streptococcus mutans ATCC 25175 strains.
| Ethanolic extract concentration |
|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% |
|---|
| Mean | 9.00 | 11.60 | 13.90 | 16.10 | 17.30 | 21.50 | 23.60 | 27.20 |
| SD | 0.94 | 1.51 | 0.88 | 0.99 | 2.06 | 1.18 | 1.58 | 2.10 |
| ANOVA: F | 176.66 |
| P | <0.001 |
| Duncan0.05 |
The seven groups showed different effects according to concentration, although the 40% and 50% concentrations did not statistically different. Antibacterial activity was highest at 80% (27.20±2.10 mm) and lowest at 10% (9.00±0.94 mm).
[Table/Fig-4] shows a statistically significant difference (p<0.05) between the antibacterial effects on S. mutans ATCC 25175 strains of different concentrations of A. muricata L. ethanolic leaf extracts (10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80%) compared with ethanol (NR or 0 mm). All concentrations were superior to alcohol.
In-vitro comparison of the antibacterial effects of different concentrations of A. muricata L. ethanolic leaf extracts and 70% ethanol on Streptococcus mutans ATCC 25175.
| Ethanolic extract concentrations | |
|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | Ethanol |
|---|
| Mean | 9.00 | 11.60 | 13.90 | 16.10 | 17.30 | 21.50 | 23.60 | 27.20 | 0.00 |
| SD | 0.94 | 1.51 | 0.88 | 0.99 | 2.06 | 1.18 | 1.58 | 2.10 | 0.00 |
| ANOVA: F | 350.19 | |
| P | <0.001 | |
[Table/Fig-5]: Analysis of variance (ANOVA) revealed differences between the A. muricata L. extracts and chlorhexidine (p <0.001 <0.05), but a comparison of the concentrations with chlorhexidine (24.2±2.25 mm) showed similarities only for the 70% concentration.
In-vitro comparison of the antibacterial effect of different concentrations of A. muricata L. ethanolic leaf extracts and 0.12% chlorhexidine gluconate on Streptococcus mutans ATCC 25175 strains.
| Ethanolic extract concentrations | |
|---|
| 10% | 20% | 30% | 40% | 50% | 60% | 70% | 80% | Chlorhexidine |
|---|
| Mean | 9.00 | 11.60 | 13.90 | 16.10 | 17.30 | 21.50 | 23.60 | 27.20 | 24.20 |
| SD | 0.94 | 1.51 | 0.88 | 0.99 | 2.06 | 1.18 | 1.58 | 2.10 | 2.25 |
| ANOVA: F | 154.54 | |
| P | <0.001 | |
Discussion
The use of new molecules from natural products becomes increasingly important. The A. muricata L could be used in toothpastes or mouthwashes for the control of the main pathogen, S. mutans, associated with the formation of dental caries [34].
The present in-vitro study compared the antibacterial effects of different concentrations of A. muricata L. ethanolic leaf extracts (10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80%) on S. mutans ATCC 25175 strains.
This study was based on the work of researchers, who applied A. muricata extracts to bacteria, although not S. mutans, and found positive antibacterial activity for both gram-positive and gram-negative bacteria:
In the present study, significant antibacterial activity against S. mutans ATCC 25175 was demonstrated for different concentrations of A. muricata L. ethanolic leaf extract (10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80%). Measurement of the inhibition zones revealed higher activity at 80% (27.20±2.10 mm) and lower activity at 10% (9.00±0.94 mm), which was directly related to the concentration. Likewise, the 40% and 50% concentrations did not significantly differ; their inhibition halos were 16.10 mm and 17.30 mm, respectively.
Comparing all concentrations with the controls revealed that the 70° ethanol (negative control) had less activity (NR or 0 mm) than all concentrations; in contrast, the 0.12% chlorhexidine gluconate (positive control) produced greater halos than did the 10%, 20%, 30%, 40%, 50%, 60%, and 70% concentrations. Only the 80% concentration (27.20 mm) exceeded the positive control (24.20 mm).
Vit P et al., found that A. muricata contains metabolites such as tannins, alkaloids, flavonoids, saponins, acetogenins, and other chemical compounds, which cause this plant’s antioxidant activity [29].
Mithum P et al., studied the antibacterial effects of 1%, 5%, 10%, 15%, and 20% A. muricata aqueous leaf extracts against S. mutans, finding halos of 20.8 mm at the 10% and 20% concentrations. This differed from the present study, which found halos of less than 9 mm and 11 mm, respectively, at those concentrations. This finding could be explained by the different origins of A. muricata L. In addition, A. muricata L. has acetogenins that are active against microorganisms [12]. Pomper KW et al., reported that Annonaceae contain bioactive compounds within their acetogenins that give this plant its antibacterial, antiviral, antiparasitic, antihypertensive, and antimalarial effects [35].
Sun S et el., measured the antibacterial effect of 5%, 15%, and 25% A. muricata hydroalcoholic leaf extracts against S. mutans and measured inhibition halos of 7 mm at 15% and 8.7 mm at 25%. Despite the different concentrations, It was found that measures were closer in this study than at the concentrations at 10% (9 mm) and 20% (11 mm). 0.12% chlorhexidine gluconate as the positive control was used, which yielded an inhibition halo of 17 mm; this was less than that the 24.20 mm [Table/Fig-6] which was found in the present study [14]. However, this result differed from that of Mithum P et al, who found a greater inhibition halo of 25.40 mm in their positive control because they used 0.2% chlorhexidine [12].
Diameters of the inhibition halos.
The reason for the difference in the antibacterial effects of A. muricata leaf extracts on S. mutans between the findings in present study with other studies could be, as stated by Ramalingum N, that the amount of acetogenins in the plant depends on the cultivation area, the variety and the climatic conditions to which it was exposed [36].
Limitation
This is an in-vitro study, since the in vivo atmosphere is different as the same result can not be reciprocated, if the herb is used in vivo settings. In current study only the leaves of the herbs were used; the other parts of the plant like the fruits, stem, and seeds should also be tested for their antibacterial potential.
Conclusion
The 10%, 20%, 30%, 40%, 50%, 60%, 70%, and 80% concentrations of A. muricata L. ethanolic leaf extracts exhibited antibacterial effects on S. mutans ATCC 25175. The effects were directly proportional to the increases in concentration. All concentrations of A. muricata L. ethanolic leaf extracts showed antibacterial effects on S. mutans ATCC 25175 compared with 70% ethanol. These results demonstrated that A. muricata L can be used as an effective antibacterial agent and could be widely applied in dental materials and to help fight dental caries.