Case Report
A 15-month-old baby girl presented to the paediatric emergency with a large swelling and bluish discolouration over the lower back present since birth. This swelling had been gradually increasing in size. There was history of intermittent bleeding which would stop spontaneously from another smaller hyper pigmented nodule on the right lower back. The clinical history included gross developmental delay with inability to walk or crawl or speak any word distinctly. The antenatal and perinatal history had been uneventful with the child born at full term through vaginal delivery in a hospital with a normal Apgar score at birth. The child had a normal older sibling.
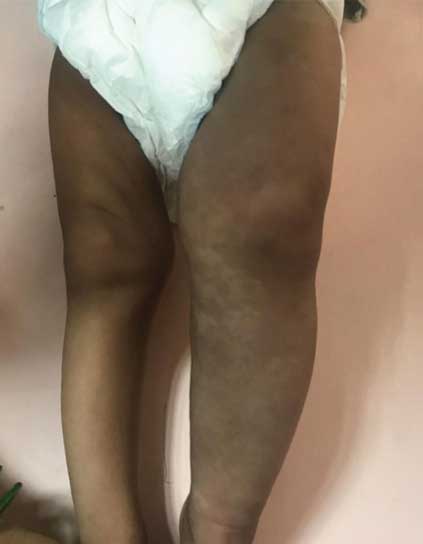
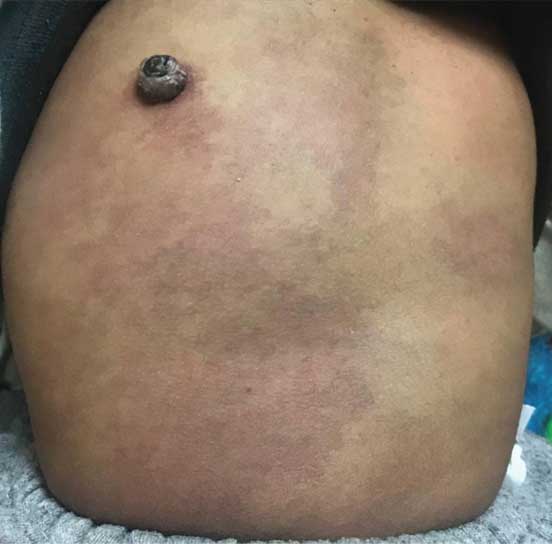
On clinical examination the child had facial dysmorphism with enlarged head (circumference of 68 cm), frontal bossing, hypertelorism and low set ears. Gross and fine motor development were inadequate for age, with inability to stand without support, absent pincergrasp, and the language function had not yet developed to the bi-syllable stage. Girth of the left lower limb was increased in comparison to that of right limb with bluish discolouration and prominent subcutaneous veins on the overlying skin [Table/Fig-1a]. A large soft tissue swelling was seen on the back involving the entire left dorso-lumbar region with prominent subcutaneous veins traversing it. The swelling was extending to involve the left lateral as well as anterior chest wall. A hyper pigmented purple cutaneous nevus with surrounding erythema and intact overlying skin was seen on the right lower back (T11-T12 level), medial to the posterior axillary line [Table/Fig-1b]. Another ill-defined soft consistency swelling of size 4×4 cm was seen extending down from the nape of neck to the upper back. Girth of both upper limbs was found similar and normal. The external genitalia were normal.
Asymmetry of both lower limbs with hypertrophy of left lower limb and bluish discolouration of the skin of left thigh and upper leg is seen.
An ill marginated large soft tissue swelling involving the left dorsolumbar region with prominent subcutaneous veins and altered colour of skin. An epidermal nevus with a raw irregular surface seen on the skin of the back, at T12 level, a hand span to the right and lateral to the midline. The skin surrounding the nevus is hyper pigmented.
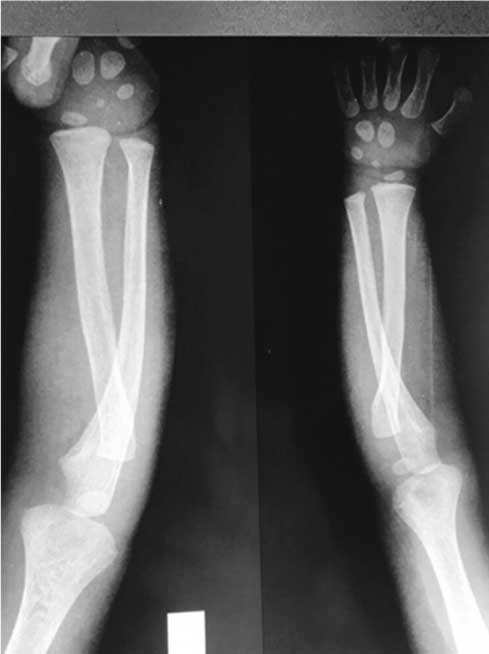
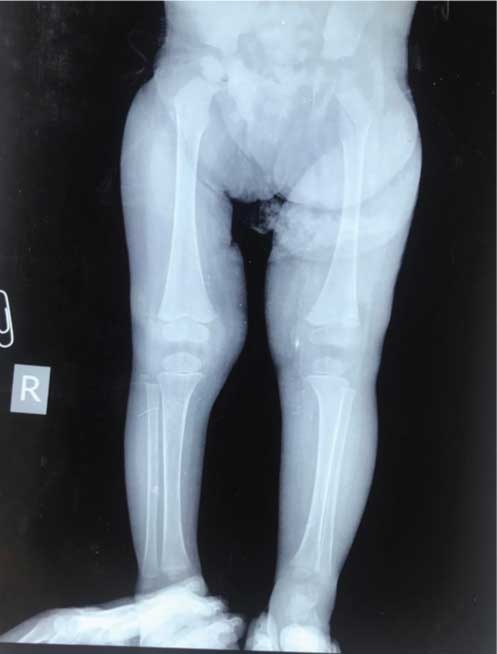
The blood work revealed low haemoglobin count of 6.9 gm% with a reduced platelet count of 60000/mm3 leading to a clinical suspicion of consumptive coagulopathy. A skeletal survey with X-rays of bilateral lower limbs, upper limbs and dorsolumbar spine was done. Bone age was seen to be greater than the chronological age and corresponded to 4 years with visualisation of ossification centers for carpal bones-Triquetral, Capitate, Hamate and Lunate in Skiagram of elbows with wrists and bilateral hands [Table/Fig-1c]. AP Skiagram of lower limbs demonstrated hypertrophy of the soft tissues and muscles of left lower limb [Table/Fig-1d]. Lower limb sonography performed for evaluation of the hemi hypertrophy demonstrated increased thickness of subcutaneous tissues along with increased echogenicity of muscles of left lower limb. Lower limb Doppler however revealed normal flow in the arteries and veins.
X-ray bilateral elbows with wrists and hands showing premature appearance of ossification centers for carpal bones.
X-ray bilateral lower limbs showing hypertrophy of soft tissues of the left lower limb.
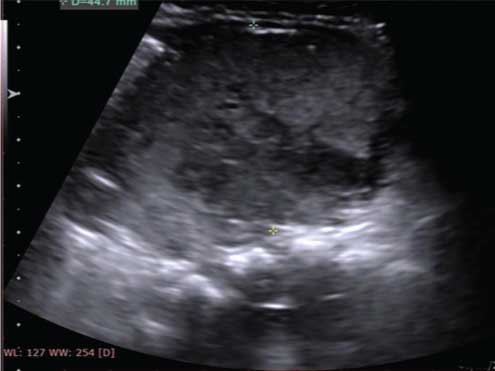
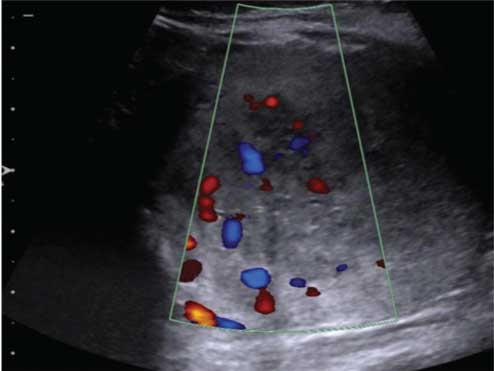
USG of the swellings at the nape of neck and the dorsolumbar region demonstrated ill-defined margins with increased internal echogenicity, consistent with lipomatous nature, without any vascularity or cystic content. On abdomen-pelvic sonography, a well-defined heterogeneously hypoechoic solid mass of size 4.7 cm (AP) × 6 cm (TR) × 4.4 cm (CC) was detected in the lower abdomen and pelvis [Table/Fig-2a]. The mass was separate from the uterus and adnexa and demonstrated central vascularity on colour Doppler [Table/Fig-2b]. Non-Contrast CT abdomen to evaluate the mass showed it to be retroperitoneal, relatively well marginated, solid, extending from left lower abdomen into the pelvis with displacement of bowel loops to the right and anteriorly.
A well marginated echogenic solid mass with small cystic areas seen in the mid lower abdomen, extending to the pelvis, but not arising from the uterus, adnexa or bladder.
Colour Doppler images shows significant vascularity within the mass.
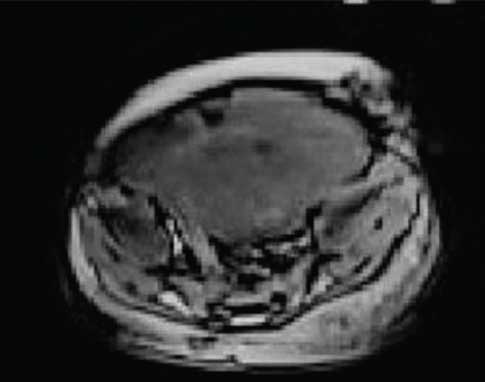
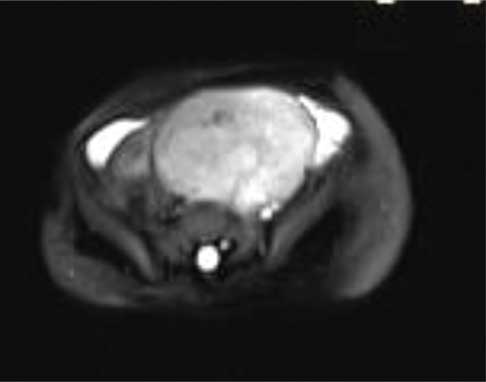
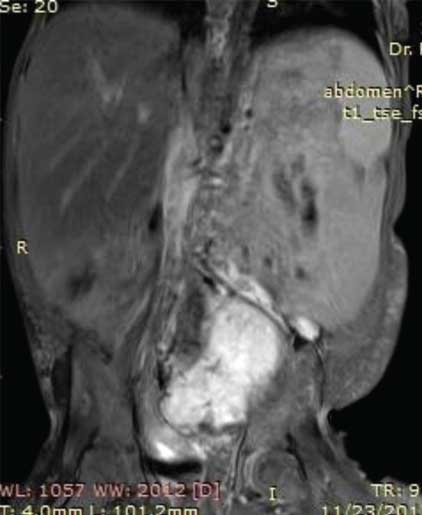
On MRI abdomen, mass was visualised in the lower abdomen, dipping into the false pelvis to the left of midline, was solid with few foci of calcification, isointense to hyperintense to muscle on T1 images and hyperintense to muscle on T2 images [Table/Fig-3a,b]. On sagittal and coronal MR the mass was seen to be pre-vertebral and retroperitoneal, with intense homogenous enhancement indicating its vascularity, and was clearly separate from the uterus and ovaries [Table/Fig-4a]. Based on the imaging characteristics, we opined the mass to be immature germ cell retroperitoneal tumour.
Axial T1 weighted VIBE MR Image demonstrates a T1 hyperintense solid mass occupying the lower abdomen and left side of pelvis. The mass is separate from the uterus and ovaries.
Axial T2 weighted MR image show the mass to be retroperitoneal in location with heterogeneously hyperintense signal intensity. The mass is seen abutting the left internal iliac artery and vein which are enlarged in comparison to the right iliac vessels. There is significant associated hypertrophy of the subcutaneous and muscular tissues of the leftthoraco-abdominal walldorsally and ventrally.
Post gadolinium Coronal T2 MR Image demonstrates intense enhancement of the mass with displacement of the adjacent bowel loops.
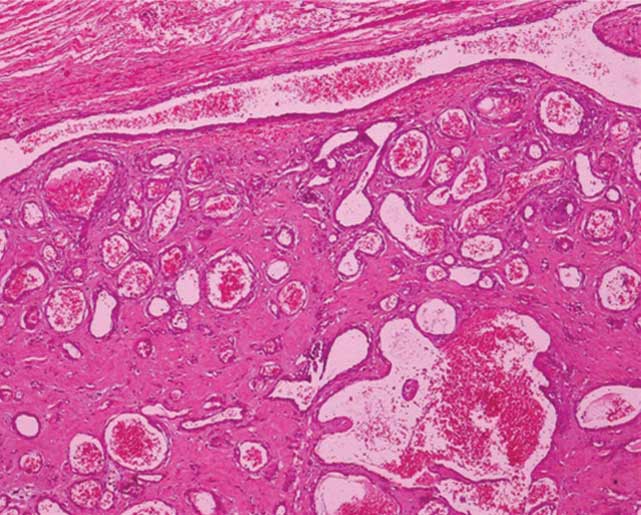
A diagnosis of Proteus syndrome was made on clinico-radiologic correlation in view of the clinical findings of unilateral limb hypertrophy (correlated on X-rays to be hemihypertrophy of the soft tissues of left lower limb, i.e., the thigh and leg along with premature appearance of carpal bones ossification centers), soft tissue swelling and enlargement in the dorsolumbar region (correlated on ultrasound imaging to be subcutaneous lipomas), an epidermal nevus, characteristic facial dysmorphism, history of delayed motor development, along with presence of a retroperitoneal tumour. The child was taken up for surgery and the tumour was intra-operatively confirmed to be retroperitoneal. Macroscopic specimen resected had the appearance of a grey white globular encapsulated tumour with multiple areas of haemorrhage. On microscopic examination, the biopsy specimen was seen to be composed of large dilated cystic appearing vessels with thin walls, within a stroma showing patchy fibrosis and hyalinization. The vascular spaces were lined by a single row of endothelial cells with some spaces showing thrombi and calcification and focal areas of back to back arrangement of vascular spaces. The histopathology report revealed cavernous haemangioma [Table/Fig-4b]. The other differentials for syndromes with cutaneous lesions and hemihypertrophy namely Bannayan Riley Ruvalcaba syndrome, Klipppel Trenauny syndrome and Neurofibromatosis 1 were considered and excluded. Bannayan-Riley-Ruvalcaba syndrome was excluded because hemimegalencephaly and gonadal or retroperitoneal tumours are features of Proteus syndrome and not of Bannayan-Riley-Ruvalcaba syndrome. Klipppel Trenauny syndrome was ruled out since limb hypertrophy was not associated with cutaneous port wine stain. Absence of cutaneous stigmata of neurofibromatosis 1 namely café au lait spots, axillary freckling and Lisch nodules as well as absence of plexiform neurofibromas and intracranial tumours removed Neurofibromatosis 1 from a probable differential.
Histopathology of microscopic specimen shows circumscribed proliferation of medium to large thin-walled dilated vascular channels lined by flattened endothelial cells. Focal areas showing intercommunicating vascular channels producing sinusoidal pattern were also noted with occasional intravascular thrombi. The surrounding stroma showed hyalinization and focal calcification. There was no cytologic atypia or brisk mitotic activity. A diagnosis of cavernous haemangioma was rendered. (H&E stain, x100)
The present case demonstrated features typical of Proteus syndrome with the soft tissue hypertrophy in one limb with subcutaneous dorsolumbar lipomas and vascular tissue proliferation to form a cavernous haemangioma. However, the unusual features encountered in this case are the skeletal manifestations in the form of bone age being more than the chronological age (carpal bone ossification centers had appeared at 15 months) and presence of associated ipsilateral hemihypertrophy of soft tissues of anterolateral abdominal walland ipsilateral dorsolumbar region. The present case is unique not only because it is extremely rare but also because although visceral vascular malformations are a component of this syndrome, a retroperitoneal cavernous haemangioma has not been described as part of Proteus syndrome, to the best of our knowledge.
Discussion
Proteus syndrome is a multisystem disorder of post natal growth dysregulation characterised by asymmetric disproportionate partial overgrowth of the body which may affect the soft tissues or bones of a limb, a digit, or involve any viscera; in combination with cutaneous or visceral vascular malformations [1]. The syndrome is unique because of abnormal growth of the musculoskeletal, cutaneous and connective tissues with concomitant vascular tissue proliferation to form hematomas, venous ectasias or varicosities in any part of the body including the viscera. Organomegaly develops as a consequence of visceral hypertrophy and abnormal soft tissue growth also leads to development of tumours of ovary, testes, parotid gland or meninges. The disorder named after the Greek god of the seas who is known to change form, was first described in 1979. Proteus syndrome is rare with an incidence of only 1:1,000,000 and manifests in the paediatric population with a male preponderance [2]. It is mostly believed to be a genetic autosomal dominant condition of somatic mosaicism caused by a germ line PTEN mutation affecting the AKT-1 gene [3]. However, there have been reports challenging this association as no intra-exonic mutations were identified in some patients with features of Proteus syndrome.
Imaging is essential for accurate diagnosis of the multi-system and visceral involvement associated with this condition, to detect neurological changes. Imaging modalities USG, CECT and contrast enhanced MRI help in differentiating Proteus syndrome from other hemihypertrophy syndromes and also to detect the tumours known to be associated with this condition. Since the disease is progressive, an accurate diagnosis is important for surgical and orthopedic interventions at appropriate time.
This rare paediatric disorder has diverse cutaneous, connective tissue, musculoskeletal, vascular system and visceral involvement and clinically manifests as abnormal segmental musculoskeletal overgrowth, along with development of subcutaneous masses and vascularmal formations. The condition comes to light early in infancy, the presenting complaint being disproportionate enlargement of an entire limb or one half of the body or merely of a digit. Asymmetric growth, defined as growth at a rate greater than that of other body parts must always be ascertained before labeling a patient with a diagnosis of Proteus syndrome. Facial dysmorphism in the form of a long face, depressed palpebral fissures, depressed nasal bridge and hypertelorism are often associated. Dark freckles on the face are found in males. Asymmetric overgrowth affects the cutaneous tissue as well, with development of the classic cerebriform scaly appearing nevus [4]. There is simultaneous abnormal proliferation of subcutaneous adipose tissue with development of lipomas. Cutaneous overgrowth is typically described on the plantar surface of foot while lipomas may be found distributed in the torso, over the extremities or on the back [5].
Haemangiomas, venous ectasias, Arteriovenous malformations or varicosities are part of vascular tissue overgrowth; the location of these may be cutaneous (in the form of a nevus in epidermis), subcutaneous, intracranial or within any abdominal viscera such as spleen or liver. The epidermal nevus is commonly found at the nape of neck or the dorsal surface of the hands, and typically appears brown or brown black in colour, slightly raised with a rough texture and faint veins coursing through it [6]. The most concerning feature of Proteus syndrome is development of facial, intracranial or abdominal tumours as part of tissue hypertrophy. The association with tumours gives serious prognostic connotation to this unusual syndrome. Prognosis of Proteus syndrome is by and large poor because of the progressive nature of the disease with premature death occurring due to sequelae of vascular tissue hypertrophy.
The myriad characteristics of this disorder that simultaneously targets so many systems can be best diagnosed by radiological modalities like USG, CECT or MRI. The plain radiograph is a very useful part of imaging that detects the asymmetric soft tissues and long bones hemi hypertrophy. Hypertrophy may affect a single digit in some patients while in others asymmetric growth may extend to involve the vertebrae with enlarged dysplastic vertebral bodies and scoliosis. Macrodactyly, polydactyly, and syndactyly may be the first skeletal changes that lead the clinician to suspect Proteus syndrome. Bony overgrowth associated with this condition can result in osteomas, osteochondromas, and cranial exostoses. In some patients, peripheral bones may not develop at all. Limb length discrepancy has been described. Calvarial thickening with unilateral hyperostosis and dolichocephaly can be detected on X-ray skull. Skiagram of the knees may show genu valgum [7]. The skeletal changes in the long bones include thin cortices with prominent trabeculae and a demineralized appearance. Cartilage calcification is an early association resulting in degenerative joint changes. As the age of child advances, due to disproportionate growth, deformities such as kyphoscoliosis or genu valgum develop, resulting in lot of discomfort.
Organomegaly is the visceral manifestation of abnormal growth and sonography demonstrates nephromegaly, splenomegaly or gastromegaly. Subcutaneous lipomatous overgrowth causes asymmetry in the anterior or posterior body wall. Doppler imaging is very useful to detect the extent of vascular malformations and to diagnose Deep vein thrombosis which is a potential life threatening complication that can result in pulmonary embolism and respiratory failure. Pulmonary tissue hypertrophy results in development of bullae, lung cysts and emphysema with respiratory distress. HRCT chest is helpful to identify these changes. Intestinal polyposis can be seen as part of tissue overgrowth. CT and MR Enterography are effective modalities for diagnosing intestinal polyposis.
Neurological involvement in Proteus syndrome has an incidence of 40%. MR is the best modality to diagnose the neurological changes of macrocephaly, hydrocephalus and hemimegalencephaly. The latter may affect a part of, or an entire cerebral hemisphere. MRI effectively diagnoses lissencephaly, schizencephaly, pachygyria, polymicrogyria, callosal dysgenesis as well as white mater cysts that can occur sporadically in parietal, frontal and temporal lobes. Cortical dysplasias are also seen as part of CNS involvement. Cerebral vascular involvement leads to cavernous venous malformations that are best demonstrated on contrast enhanced MR. The nidus, artery supplying it and draining vein are identified on CE MR [8]. CNS changes result in delayed development, hypotonia and epileptiform seizures. Refractory epilepsy often occurs in children of Proteus syndrome due to hemimegalencephaly, neuronal migration and callosal dysgenesis. The association of infantile spasms, myoclonus, and partial epilepsy in newborn infants with extensive migration disorders is labeled as Ohatahara syndromeand can be detected on MR or CT [9]. Abnormal tissue proliferation in the CNS can result in tumours like lipomas and meningiomas which are often multiple.
A 19% of tumours in Proteus syndrome can be malignant. MRI due to the excellent soft tissue resolution and anatomic multiplanar capability is gaining high acceptability as the imaging modality of choice for diagnosis of tumours associated with the syndrome. Genital tract tumours encountered may be ovarian epithelial cystadenoma, testicular or ovarian germ cell tumours and epididymal cysts [10]. Lipomas appear as uniformly echogenic poorly marginated masses on sonography. Monomorphic adenomas of parotid gland and ovarian tumours are included as specific diagnostic criteria for Proteus syndrome [11].
Although the syndrome is distinct due to sporadic and mosaic distribution of clinical features with disparate body parts involvement, the diagnosis often becomes clinically challenging. There is an overlap and close similarity between some clinical features of Proteus syndrome and the equally rare Banyan Riley Ruvalcaba syndrome, another hamartomatous polyposis syndrome hat too manifests with macrocephaly, delayed development, lipomas, haemangiomas and intestinal polyps. Clinically, asymmetric growth is a characteristic feature of Proteus syndrome while clinical characteristics in Bannayan-Riley-Ruvalcaba syndrome are presence of speckled macules on the penis in a male child, pectus excavatum and myopathy with involuntary muscle movement [12]. However, hemimegalencephaly and gonadal or retroperitoneal tumours are seen in Proteus syndrome and not in Bannayan-Riley-Ruvalcaba syndrome and identification of these changes is only possible by imaging which is therefore important in differentiating the two. Clinically, hemi hypertrophy with vascular malformations may be seen in other syndromes such as Klipppel-Trenauny syndrome, Hemi hyperplasia-multiple lipomatosis syndrome, Mafucci syndrome and Neurofibromatosis 1. Radiological imaging aids in this differentiation by detecting the internal characteristics of each condition. The typical triad of cutaneous port wine stain, vascular malformations and limb hypertrophy in a child are characteristic of Klipppel Trenauny syndrome and Colour Doppler or MRI are both effective in diagnosis of the low flow venous and lymphatic malformations with extensions into the muscle compartments. Imaging assists in differentiating Neurofibromatosis 1 from Proteus syndrome by demonstrating the characteristic ‘bare orbit sign’ on X-ray skull and the optic gliomas, meningiomas or plexiform neurofibromas on MRI.
The management of Proteus syndrome is largely symptomatic and involves tackling the multi-system complications. Medical management includes antiepileptic medicines, warfarin or heparin; the skeletal deformities require repeated orthopedic interventions, while developmental delay can improve with palliative surgery like hemispherectomy for hemimegalencephaly.
The unique features in this patient of Proteus syndrome are: the bone age being more advanced than the chronological age, the hemihypertrophy of ipsilateral anterolateral abdominal wall associated with limb hypertrophy and the detection of cavernous haemangioma in the retroperitoneum. Many retroperitoneal tumours have been described with Proteus syndrome but a cavernous haemangioma due to vascular tissue hypertrophy in the retroperitoneum is an unusual manifestation and not been mentioned in literature to the best of our knowledge.
Conclusion
Imaging has a large role in the diagnosis of the myriad involvements in Proteus syndrome. It is important for the paediatrician to include radiological imaging as part of the clinical work up of a child presenting with hemi hypertrophy in order to know the extent of occult and overt manifestations of this condition. The paediatrician and radiologist both must be aware of this unusual disorder. The condition may be overlooked or misdiagnosed if clinico-radiologic correlation is omitted. Imaging identifies the CNS, pulmonary, vascular and skeletal changes of Proteus syndrome and eliminates the other hemihypertrophy syndromes that are associated with vascular malformations.
[1]. Cohen MM, Proteus syndrome-an updateAm J Med Genet 2005 137 C:38-52.10.1002/ajmg.c.3006316010681 [Google Scholar] [CrossRef] [PubMed]
[2]. Alves C, Acosta AX, Toralles MB, Proteus syndrome: Clinical diagnosis of a series of casesIndian J Endocrinol Metab 2013 17(6):1053-56.10.4103/2230-8210.12262124381883 [Google Scholar] [CrossRef] [PubMed]
[3]. Zhou X, Hampel H, Thiele H, Gorlin RJ, Hennekam RC, Parisi M, Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromesLancet 2001 358:210-11.10.1016/S0140-6736(01)05412-5 [Google Scholar] [CrossRef]
[4]. Talari K, Subanna PKA, Amalnath D, Suri SD, Proteus syndrome: A rare case reportIndian J Hum Genet 2012 18(3):356-58.10.4103/0971-6866.10803623716948 [Google Scholar] [CrossRef] [PubMed]
[5]. Beachkofsky TM, Sapp JC, Biesecker LG, Darling TN, Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndromeJ Am Acad Dermatol 2010 63:799-804.10.1016/j.jaad.2009.12.01220709429 [Google Scholar] [CrossRef] [PubMed]
[6]. Tan WH, Baris HN, Burrows PE, Robson CD, Alomari AI, Mulliken JB, The spectrum of vascular anomalies in patients with PTEN mutations: implications for diagnosis and managementJ Med Genet 2007 44:594-602.10.1136/jmg.2007.04893417526801 [Google Scholar] [CrossRef] [PubMed]
[7]. Jamis-Dow CA, Turner J, Biesecker LG, Choyke PL, Radiologic manifestation of Proteus syndromeRadiographics 2004 24:1051-68.10.1148/rg.24403572615256628 [Google Scholar] [CrossRef] [PubMed]
[8]. Johnson CM, Navarro OM, Clinical and sonographic features of paediatric soft-tissue vascular anomalies part 2: vascular malformationsPaediatric 2017 47(9):1196-208.10.1007/s00247-017-3906-x28779187 [Google Scholar] [CrossRef] [PubMed]
[9]. Ogrodnik M, Sadowski Jóźwiak S, Neurological manifestations of Proteus syndrome- review of the literatureChild Neurology 2016 49:47-53.10.20966/chn.2015.49.353 [Google Scholar] [CrossRef]
[10]. Bastos H, da Silva PF, de Albuquerque MA, Mattos A, Riesgo RS, Ohlweiler L, Proteus syndrome associated with hemimegalencephaly and Ohtahara syndrome: Report of two casesSeizure 2008 17:378-82.10.1016/j.seizure.2007.11.00118082431 [Google Scholar] [CrossRef] [PubMed]
[11]. Gordon PL, Wilroy RS, Lasater OE, Cohen MM Jr, Neoplasms in proteus syndromeAm J Med Genet 1995 57:74-78.10.1002/ajmg.13205701177645604 [Google Scholar] [CrossRef] [PubMed]
[12]. Turner JT, Cohen MM, Biesecker LG, Reassessment of the Proteus syndrome literature: Application of diagnostic criteria to published CasesAm J Med Genet A 2004 130A:111-22.10.1002/ajmg.a.3032715372514 [Google Scholar] [CrossRef] [PubMed]