Diabetes mellitus (DM) is a multi-system, lifelong metabolic disease requiring comprehensive management provided by Health Care Professionals (HCPs) [1]. The complex nature of this chronic disease requires management strategies and frequent follow-up via biochemistry testing for monitoring glycaemic control to decrease incidence rate of long-term complications [2-4].
However, centralised laboratory testing entails a long processing time and may lead to potential medical risks; hence, a rapid clinical decision-making support is highly important for modern glucometer development towards an agile, efficient and accurate analysis, such as POCT [5].
POCT, also known as near-patient or bedside testing, is defined as any testing performed by HCPs on-site at the time of consultation, which allows instant availability of results to make immediate and informed decisions about patient care [6-8]. Recently, the POCT Blood Glucose Monitoring Systems have been found to have a positive impact on the management of patients with DM [9]. These POCT Systems should have the following characteristics like Self-Monitoring of Blood Glucose (SMBG) Systems, which includes: (i) applicability for blood sampling from different body sites, such as fingertips, palms, arms, veins; (ii) wide range of haematocrit (HCT) levels, including neonates (low HCT) and dialysis patients (high HCT), no interference in glucose measurements of POCT Systems; (iii) suitability for patients with any blood-oxygen level; (iv) automatic upload of testing results to the central laboratory information management system via Wireless Fidelity (WiFi) [10-13].
Cathay General Hospital recently implemented the Rightest POCT, GM700 Pro, for inpatient bedside glucose monitoring in all hospital systems. Therefore, to assure the quality of POCT, a domestic, large patient scale (N >1,000) study was conducted for evaluation of POCT. In addition, the application of POCT to outpatient/emergency care workflow, especially for pre-meal and post-meal blood glucose test or gestational blood glucose test was also assessed. Due to insufficient direct data to reflect how work efficiency can be improved by integrating POCT in outpatient/emergency medical service, a parallel test was carried out to compare the TAT of the central laboratory and POCT testing.
Materials and Methods
The retrospective study was conducted in the Outpatient/Emergency Biochemistry Laboratory Department of Cathay General Hospital, which is one of the 19 medical centres nationwide. The study was approved by the Hospital Ethics Committee (CGH IRB number: CGH-CS105004).
The inclusion criteria were: age over 20 years, admission to outpatient clinics, and prompt biochemistry blood test requirement from November 2016 to November 2017. The prompt biochemistry analysis was generally processed and reported to the patient on the same day. All laboratory measurements were performed by trained laboratory staff based on Standard Operating Procedures (SOP) in an accredited laboratory.
Procedure
The consenting subject checked-in at the outpatient phlebotomy counter and time was automatically recorded. The subject underwent a venipuncture procedure. Venous blood was sampled in heparinised tubes with gel separator (Becton Dickinson, USA) and POC glucose was measured using the Rightest GM700 Pro. The results were plasma-equivalent glucose. The data were validated right after the test. Immediately after POCT, the plasma was separated from cells by centrifugation (3500 × g, 3 min). Automated hexokinase assay, Beckman Coulter BC-AU680, with accreditation according to ISO15189 standard, was used as the reference standard laboratory procedure for venous plasma glucose measurement.
Two widely accepted standards for blood glucose meters ISO 15197:2013 (for self-monitoring) and USFDA Prescription Point-of-Care Use (for hospital use) were adopted to evaluate the accuracy of the Rightest GM700 Pro. ISO 15197:2013 specified that 95% of the individual meter results should fall within ±15 mg/dL of the reference measurement procedure at glucose concentrations <100 mg/dL, and within ±15% at glucose concentrations ≥100 mg/dL. The FDA suggested that a glucometer sufficiently accurate to use by HCPs, must be able to meet these two criteria [6,8]:
At <75 mg/dL, 95% of results should be within ±12 mg/dL; at ≥75 mg/dL, 95% of results should be within ±12%
At ≤75 mg/dL, 98% of results should be within ±15 mg/dL; at >75 mg/dL, 98% of results should be within ±15%
The TAT to the total period from receipt of the clinician’s order, collection of specimens, sample analysis, data verification and results delivery to review of Hospital Information System (HIS). The TAT of POCT and CLT was calculated according to the automatic time capture in the Rightest GM700 Pro, bio-analyser and Laboratory Information System (LIS). To avoid human errors and bias, there was no manual data entry, whether time or glucose measurement. The TAT data of each method are presented as mean±SD. The bi-hourly distribution of TAT is calculated.
Statistical Analysis
Agreements between the Rightest GM700 Pro and CLT (reference method) were presented as mean±SD. Pearson’s correlation and Passing-Bablok regression were used for the analysis of method. Bland-Altman analysis was used to determine the agreement between POCT and CLT glucose results.
Results
The total number of recruited patients was 2,109. We did not include incomplete data (N=297, 14.0%) due to machine breakdown, human errors and no Wi-Fi or internet connection. A total 1,812 subjects (β=80%) were included for analysis. The mean age was 59.7±16.3 years and the mean HCT was 36.7%±5.3%. Baseline characteristics of study patients are presented in [Table/Fig-1].
Baseline Characterisics of the participants under study.
| Factors | Values |
|---|
| Age (years) | 59.7±16.3 |
| Female gender, n (%) | 1098 (60.6) |
| Haematocrit (%) | 36.7 (23.2-51.2) |
| Mean CLT Plasma Glucose (mg/dL) | 161±63 (50-558) |
| Mean Rightest GM 700 Pro Whole Blood Glucose (mg/dL) | 156±62 (46-507) |
Data represented as Mean±SD; overall range in parentheses
The mean venous central lab blood glucose test was 161±63 mg/dL. The mean Rightest GM700 Pro venous blood glucose was 156±62 mg/dL. Mean measurement difference (bias) between the Rightest GM700 Pro and central lab blood glucose test is presented in [Table/Fig-2]. The Pearson’s correlation was 0.99 (p-value <0.05), demonstrating a significant agreement between the Rightest GM700 Pro and central lab blood glucose test.
Comparison between central laboratory test and Rightest GM700Pro blood glucose measurements.
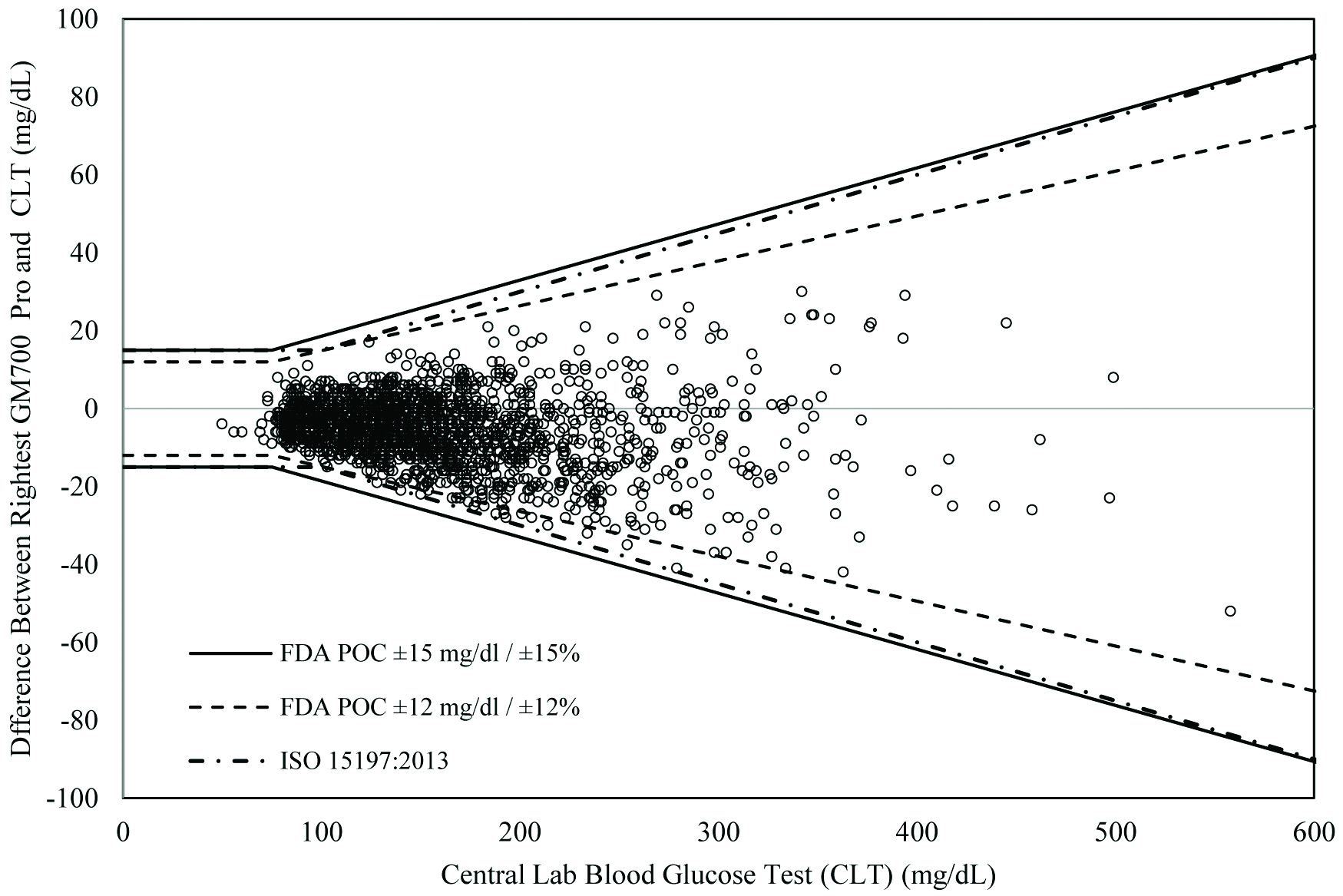
Over the whole range, the Rightest GM700 Pro displayed blood glucose values that were approximately 5.5 mg/dL (95% CI: 5.1 to 5.9 mg/dL) lower than CLT values. We found that 100.0% (1812/1812) of the individual Rightest GM700 Pro results were within the International Standards Organisation (ISO) accuracy limits (ISO 15197:2013); 97.2% (1761/1812) of the individual Rightest GM700 Pro results were within the FDA Prescription Point-of-Care Use (POC) (±12 mg/dL and ±12%) accuracy limits and 100.0% (1812/1812) of the individual results were within the FDA POC (±15 mg/dL and ±15%) accuracy limits [Table/Fig-2].
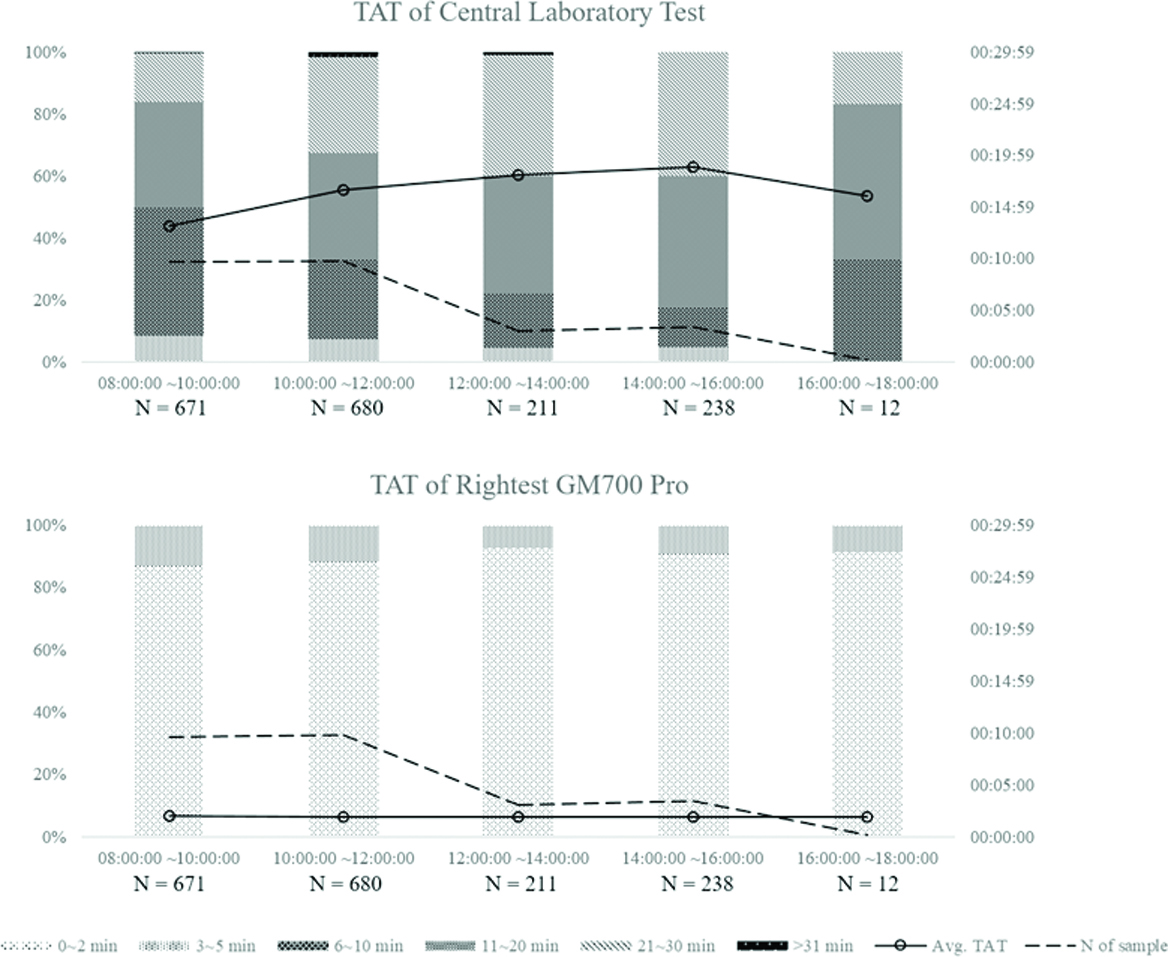
Meanwhile, the mean turnaround time for Outpatient/Emergency Biochemistry Department samples was 15.8±6.9 minutes, ranging from 5.0 to 30.9 minutes from the time the patient checked in at the phlebotomy counter to the time the reports were dispatched. Approximately, 71.9% of emergency samples in CLT were reported in less than 20 minutes and 99.2% of emergency samples were reported within 30 minutes. The mean test was 2.0±0.7 minutes, ranging from 0.2 to 3.5 minutes. Without pre-analytical task, 88.8% of blood glucose tests by POCT were reported in two minutes and 100.0% of reports were issued within 5 minutes. [Table/Fig-3] shows the distribution of the bi-hour TAT and despite the increasing number of samples, using POCT (Rightest GM700 Pro) for blood measurement could constantly keep the total testing cycle down within 2 minutes. TAT of CLT, on the other hand, was affected by the arrival of a number of samples within a short period of time. When the number of samples increased, the processing time also increased (average of 4.9 minutes). However, the prolonged effect did not resolve right after the sample number decreased, but instead, lasted for four hours. The CLT analysis reached a bottleneck at noon and returned to regular throughput at 16:00:00. About two-fifths of samples were processed longer than 20 minutes from 10:00:00 to 16:00:00. This indicates that each patient, who returned to the clinic for follow-up had to spend an additional 20 minutes for the laboratory report, compared with POCT integrated outpatient visits. It is clear that the results were significantly (p<0.0001) faster with POCT than with CLT. The implementation of POCT in the Department test workflow can increase efficiency by 7 times on average blood glucose analysis [Table/Fig-3].
Distribution of the bi-hour TAT of CLT and Rightest GM700 Pro.
Discussion
The Rightest POCT system has been adopted to facilitate a timely intervention for fluctuating glycaemia in several medical mechanisms, once the GM700 Pro connects to the HIS, it can automatically receive and verify medical orders, provide measurement schedule and perform automatic recording for sample status and its irregularities. As a bedside glucose testing system, it has to have an ISO-qualified or laboratory standard clinical accuracy and highly efficient TAT report delivery. For this purpose, this study evaluated system accuracy and TAT of blood glucose testing between POCT and CLT for patients who needed prompt biochemistry testing.
The study proved that the POCT can decrease the average laboratory report delivery time by approximately 13.5 minutes per patient compared with the CLT. Depending on the time that a patient registers at the phlebotomy counter, the waiting time for blood glucose report analysed using POCT, can be made shorter to 30 minutes compared to using CLT. In addition, adopting POCT for a routine blood glucose check-up can significantly trim down the workflow by collaborating with several work station into one step on one system. Patient registration, sample collection, pre-analysis process (centrifugation and plasma collection) and post-analysis process (data verification and report delivery) can be completed using the Rightest GM700 Pro. Laboratory workforce can be redesigned and highly skilled laboratory personnel can be utilised for other urgent tests [14,15].
According to the predefined pathway of both POCT and CLT for glucose testing, the TAT of each test analysed using POCT should be at least 5 minutes faster than using CLT. However, we found that 2% of tests were inconsistent. The TAT of POCT was only faster than CLT by three minutes. Based on interviews with personnel, we conclude two possible causes. Firstly, wireless connection was temporarily inaccessible and resulted in transfer delay. Secondly, there was inconsistent pre-analytical sample preparation. Thus, we suggest medical institutions that choose to use POCT for inpatient care should develop SOPs and improve network equipment in the near future.
To verify whether the Rightest GM700 Pro can produce clinically accurate blood glucose results to support medical decision-making, we referred to the accuracy criteria specified in the FDA’s POCT draft guidance and ISO 15197:2013 for the evaluation [16,17]. The results showed that this device meets both numerical accuracy criteria. However, during the study and training period, we found that failure of HCP’s in following proper operating procedures resulted in test errors in the workflow even if an accurate system was used. For example, pre-analytical error results from poor sampling or strip storage could cause inaccuracy or improper data-handing could result in post-analytical errors [13]. In order to ensure the test quality prior to operation, relevant knowledge and training related to the device must be provided to HCPs.
Although CLT remains the gold standard in clinical practice, its main disadvantage is that its analysis is remotely located away from the patient, thereby increasing the TAT for starting treatment based on the testing result. The Rightest POCT system is regarded as a relatively accurate form of glucose management and has also been considered small, simple to use, easily interpretable and stable, which can effectively increase the speed and frequency of glucose testing. Therefore, if hospitals are able to develop the SOPs, quality assurance and training protocol for this system, there will be benefits in terms of improving treatment adherence, patient satisfaction and diabetes management.
Limitation
Though the overall number of subjects exceeded the minimum requirement addressed in ISO 15197:2013 and USFDA POC, the sample proportion of low glucose concentration were few. In addition, the sample variation was limited, and cohort studies in different care units are required to validate the results. In addition, the cost of POCT and CLT were not compared, due to lack of specific costs to individual elements of a care process. The National Health Care Insurance has no distinct insurance coverage of these two pathways yet.
Conclusion
From the study, we conclude that people with DM or especially impaired glucose intolerance should be diagnosed using the traditional CLT procedure. However, the POCT blood glucose monitoring system is more convenient; thus, we suggest that this device be used in day care cases, outpatient clinics and general practice to improve efficiency and reduce patient waiting time.
Data represented as Mean±SD; overall range in parentheses