It has been found to be associated with outbreaks of neonatal meningitis, especially in premature infants during the first two weeks of life [4]. In adults, it can cause healthcare-associated pneumonia, endocarditis, bacteremia, meningitis, device-associated infections and skin soft tissue infections, predominantly in patients with severe underlying diseases, prolonged hospitalisation, treatment with invasive procedures, prior use of broad-spectrum antimicrobials and concomitant infections. These factors have impacted survival rates [6] with mortality rates being as high as >50% depending on the population studied [3,5,7]. Clinical and Laboratory Standards Institute (CLSI) has not provided any interpretive MIC breakpoints of antibiotics against this, making the choice of treatment difficult [8]. There is no optimal regimen for treatment, and therapy should be based on properly performed susceptibility testing. Possible regimens include vancomycin, rifampin in combination with trimethoprim-sulfamethoxazole, a fluoroquinolone, or minocycline [4].
Materials and Methods
This prospective study was conducted in the Microbiology Department of a tertiary Liver care hospital in New Delhi from January 2017- December 2018, after obtaining approval from the institutional ethics committee (IEC/2017/64/NA10). All consecutive patients with positive cultures of E.meningoseptica were included in the study.
The study included patient’s demographic data, comorbidities, culture source, type of infection, duration of hospitalisation and use of invasive procedures like central venous catheters, need for ventilator and recent surgical procedures. Infection due to E. meningoseptica was defined when a patient developed signs and symptoms of infection such as fever of >38.3°C, leucocytosis >10,000/mm3 and cultures were sent based on clinical suspicion of bacterial infections. Prior ICU stay and mechanical ventilation were considered significant when patients required the same for >48 hours before isolation of E. meningoseptica. Patients who stayed in the hospital for more than 10 days before isolation of E. meningoseptica were regarded as those requiring prolonged hospitalisation. Any patient who underwent a surgical procedure (except tracheostomy) during the current hospital admission prior to isolation of E. meningoseptica was labelled to have undergone a recent surgery.
E. meningoseptica is an aerobic, non-lactose fermenting, oxidase positive, non motile GNB [Table/Fig-1]. VITEK2 system (BioMerieux Vitek; BioMerieux, Marcy I’Etoile, France) was used for genus and species level identification and antimicrobial susceptibility testing using VITEK 2 GN and AST N281 cards, respectively. CLSI did not establish definite interpretative MIC breakpoints for E. meningoseptica until 2018; thus, the MICs were interpreted based on the CLSI criteria for other non- Enterobacteriaceae (other non-Enterobacteriaceae include Pseudomonas spp. other than P. aeruginosa and other non fastidious, glucose non fermenting, Gram negative bacilli, but exclude P. aeruginosa, Acinetobacter spp., B. cepacia, B. mallei, B. pseudomallei, and S. maltophila) [8]. Vancomycin was used as reserve antibiotic as per the hospital antibiotic policy and hence isolates that were resistant to other drugs were tested for vancomycin using epsilometer gradient strips. Breakpoints for Staphylococcus aureus were used to interpret the MIC for vancomycin.
Growth of E. meningoseptica on Mac Conkey agar.
Statistical Analysis
Analysis was done using SPSS software version 22. The categorical data was represented in frequency (%) and continuous data in mean.
Results
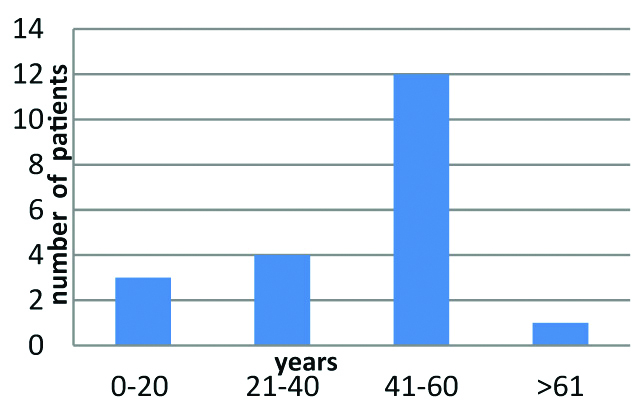
A total of 32 isolates from 20 patients were included in the study. Out of the 20 patients, 15 (75%) were males and 5 (25%) were females. Mean age was 43.05 years (range, 2-71 years). Age wise distribution of the isolates is given in [Table/Fig-2].
Age wise distribution of the isolates.
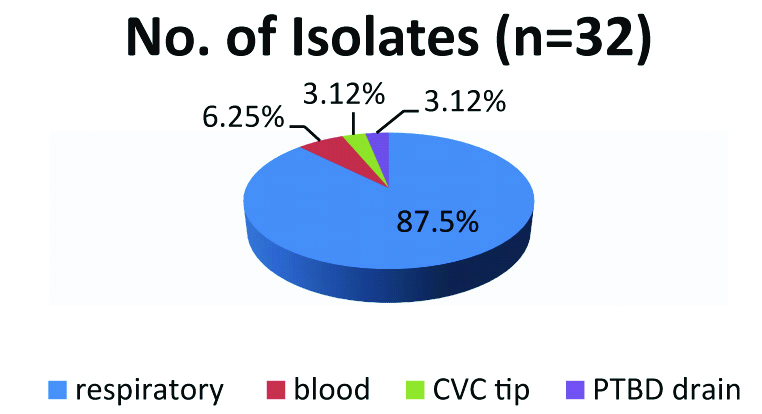
Out of the 32 isolates, E. meningoseptica was most frequently isolated from respiratory samples, 28 (87.5%). The respiratory samples included 23 isolates from non-bronchoscopic Bronchoalveolar lavage (nb-BAL), two isolates from sputum, two isolates from endotracheal aspirate and one isolate from Bronchoalveolar lavage (BAL). Two isolates (6.25%) were isolated from blood and there was an isolate each from samples CVC tip and Percutaneous transhepatic biliary drain (PTBD) [Table/Fig-3].
Sample wise distribution of the isolates.
Comorbidities associated were chronic liver disease 75% (n=15), diabetes 50% (n=10), steroid usage 45% (n=9), alcoholism 20% (n=4), cancer 10% (n=2), carcinoma of the small bowel and hepatocellular carcinoma (n=1), chronic obstructive pulmonary disease 5% (n=1). Serum albumin levels were low in all the patients [Table/Fig-4].
The possible risk factors for colonisation or infection with E. meningoseptica are listed in [Table/Fig-5]. Chronic liver disease (75%), diabetes mellitus (50%), prolonged hospital admission (55%), prior ICU stay (100%), invasive procedures like insertion of CVC (100%), need for life supports like prolonged ventilation (70%) and haemodialysis (55%). Prior to isolation of E. meningoseptica were observed frequently in the study population among the other risk factors used to evaluate patients for developing infection during their course of stay in the hospital. The mean duration of current hospital stay is 8.5 days with a range of 4-30 days. The median duration and number of antibiotics used before isolating E. meningoseptica was 16 days and four (range, 3-7), respectively.
Risk factors for colonisation or infection with E.meningoseptica (n=20).
| Risk factor | Number (%) |
|---|
| Chronic liver disease | 15 (75) |
| Diabetes mellitus | 10 (50) |
| Steroid usage | 9 (45) |
| Prolonged hospital stay | 11 (55) |
| ICU admission | 20 (100) |
| Organ transplantation | 2 (10) |
| Prolonged ventilatory support | 14 (70) |
| Recent surgery | 6 (30) |
| Use of 3 or more antibiotics | 20 (100) |
| Central line insertion | 20 (100) |
| Malignancy | 2 (10) |
| Tracheostomy | 6 (30) |
| Hypoalbuminemia | 20 (100) |
| Haemodialysis | 11 (55) |
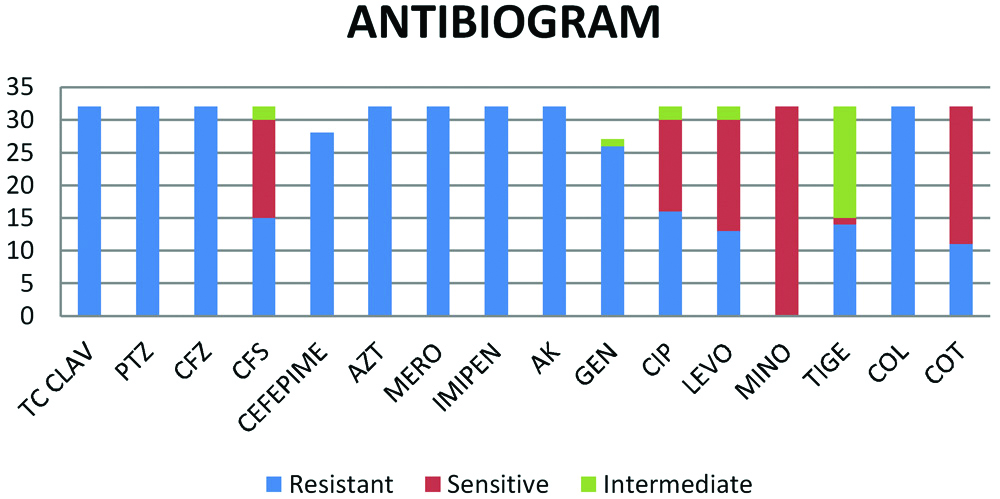
The antibiotic susceptibility of the isolates is shown in [Table/Fig-6]. Minocycline was the most susceptible drug with (32 of 32)100% sensitivity followed by cotrimoxazole 65.6 % (21 of 32), Levofloxacin 53.12% (17 of 32), cefoperazone-sulbactum 46.8% (15 of 32) and ciprofloxacin 43.75% (14 of 32). However, most of the antibiotics that were active against GNB were found to be non susceptible against E. meningoseptica.
Antibiotic susceptibility of the isolates.
TC CLAV: Ticarcillin-clavulanic acid; PTZ: Piperacillin-Tazobactum, CFZ: Cefoperazone; CFS: Cefoperazone sulbactam; AZT: Aztreonam; MERO: Meropenem; AK: Amikacin; GEN: Gentamicin; CIP: Ciprofloxacin; LEVO: Levofloxacin; MINO: Minocycline; TIGE: Tigecycline; COL: Colistin; COT: Sulfamethoxazole-trimethoprim
Nine of 20 (45%) patients died during the course of their disease, out of which 4 patients died within 1 week of isolation of E. meningoseptica. Five patients of the 20 were critically ill and left against medical advice despite explaining the need for life supports.
Discussion
Most authors have described the increased incidence of reporting E. meningoseptica causing outbreaks [5] but there have also been isolated case reports [3]. During the current study period, from January 2017-December 2018, the incidence of reporting the organism was not associated with any outbreak.
Unlike most studies where patients in their extremes of age have been more prone to the infection, [1,2] in the current study patients between the 40-60 years had maximum incidence of presentation with the mean age being 43.05 years, predominated by males (75%). This changing trend was also observed in studies done by Ratnamani MS et al., and Moore LS et al., with mean age being in the range of 50-60 years and 45 years, respectively [9,10]. In earlier reports the time of admission to acquisition of E. meningoseptica infection ranged from 50-70 days [11], but studies done by Ratnamani MS et al., and Hamza WS et al., showed that the mean duration of hospital stay to detection was early being 27 days and 5 days, respectively [6,9]. In the present study the mean duration of hospitalisation to isolation was 8.5 days.
E. meningoseptica could be found as a simple coloniser or could be the cause of symptomatic acute infections [6]. The respiratory tract was the most common site of infection of E. meningoseptica, followed by bacteraemia being the second most common presentation. In the present study, 87.5% (n=28 of 32) isolates have been from the respiratory samples with 71.8% obtained from nb-BAL. A 6.25% were isolated from blood and an isolate from CVC tip and PTBD drain. In patients with bacteraemia repeat blood culture were performed to confirm the organism and rule out contamination. The blood culture of the patients showing a growth of E. meningoseptica in CVC tip and PTBD drain did not show any growth indicating that probable colonisation of the tips could not be ruled out, this can be attributed to the fact that E. meningoseptica is known to colonise and produce biofilms [4]. Biofilm producing strains have been associated with poorer prognosis and early removal of catheter has aided in early resolution of infection caused by the organism [7].
Various reports of E. meningoseptica infection have been reported in patients who underwent bone marrow transplantation [12]. Cases have also been reported following solid organ transplant pertaining to liver, lung and kidney [12]. In this study E. meningoseptica was isolated from two patients who underwent liver transplantation.
Chronic liver disease (75%), diabetes mellitus (50%), prolonged hospital admission (55%), prior ICU stay (100%), invasive procedures like insertion of CVC (100%), Hypoalbuminemia (100%), need for life supports like prolonged ventilation (70%) and haemodialysis (55%) prior to isolating E. meningoseptica were observed frequently in the study population among the other risk factors used to evaluate patients to developing the infection while in the hospital.
Pneumonia outbreaks related to mechanical ventilation caused by E. meningoseptica have been frequently reported [6,11]. Pereira GH et al., in his observations, agreed upon the fact that patients requiring prolonged mechanical ventilation and patients transferred in with current episode of infection could be an important source of transmission [11]. There could be a possible association between contaminated water, mechanical ventilation respiratory equipment, and indwelling devices and hospital outbreaks. This could be the main reason for isolation of E. meningoseptica from respiratory samples. In the present study 70% of the patients required ventilator support necessitating tracheostomy in 5 of the 20 patients.
Prolonged hospitalisation, insertion of CVC, hypoalbuminemia, diabetes, use of multiple antibiotics for prolonged periods, haemodialysis and immunosuppresion were amongst the commonly associated risk factors that have been frequently associated with development of infection with E. meningoseptica [1,6,7,9,11,13].
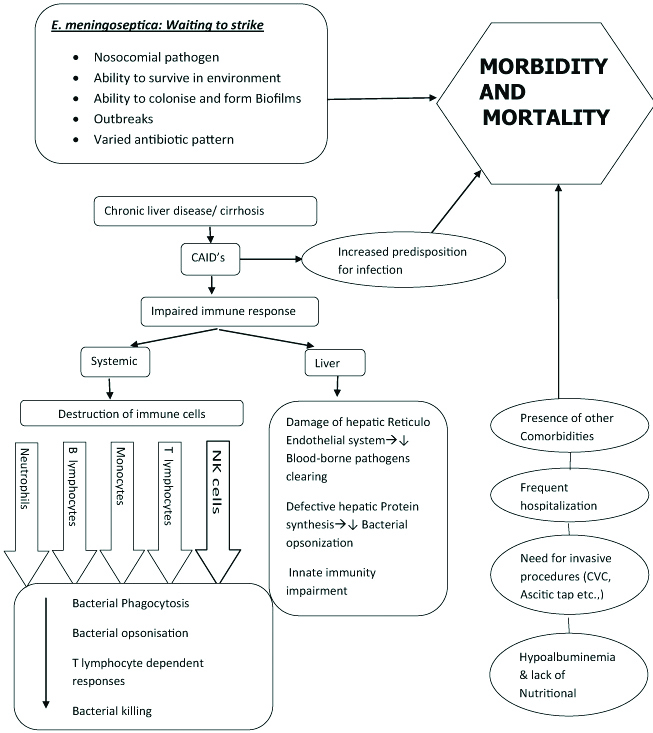
As per our knowledge, this is the first study to report that chronic liver disease could be a potential risk factor for E. meningoseptica infection. In the current study 75% of the patients with chronic liver disease/cirrhosis have developed an infection with E. meningoseptica compared to only 25% of those who did not have cirrhosis. Cirrhosis due to any aetiology disrupts the homeostatic role of liver in the body. Patients with chronic liver disease have been shown to have hypoalbuminemia, increased need for invasive procedures, and special nutritional requirements making them an immunocompromised group. Cirrhosis-Associated Immune Dysfunction (CAID’s) is a condition which refers to both immunodeficiency and systemic inflammation that occur in cirrhosis [14]. Variations in the local and systemic immunity in patients with CAID’s could lead to alterations in both innate and acquired immunity. Following which immune paralysis can set in being marked by rise in anti-inflammatory cytokines and decrease of pro-inflammatory cytokines [14,15] predisposing the individual to infection [Table/Fig-7].
Risk factor association of Chronic Liver disease with Elizabethkingia infection.
CAID’s: Cirrhosis associated immune dysfunction
Indiscriminate usage of broad spectrum antibiotics has become a habitual norm of the day for patients getting admitted to hospitals but E. meningoseptica is naturally resistant to most beta lactams and carbapenems, due to its ability to produce chromosomal metallo- betalactamases (GOB-18 and BlaB genes), which can hydrolyze most beta-lactam antibiotics and limit their usefulness as a therapeutic option [11].
Prolonged usage of multiple antibiotics has been a persistent finding before acquisition of E. meningoseptica. Moore LS et al., in their outbreak investigation of E. meningoseptica in adult critical care ICU found that 26 patients had received broad-spectrum antimicrobial drug regimes in the week preceding acquisition (Piperacillin/tazobactam or meropenem) [1,10]. Exposure to multiple antibiotics especially to carbapenems, beta lactams and colistin has been a common factor [1,9]. Joo HD et al., in his study stated that the median duration of antibiotic use was 33 days (range, 1-216 days) and number of antibiotics used before isolating E. meningoseptica was four (range, 1-11), respectively [16]. In the present study too, similar findings of prolonged multiple antibiotic usage is notable with the median duration and number of antibiotics used before isolating E. meningoseptica being 16 days and four (range, 3-7), respectively.
Current study showed that minocycline was the most susceptible drug with (32/ 32)100% sensitivity followed by cotrimoxazole 65.6% (21/32), levofloxacin 53.12% (17/32), cefoperazone-sulbactum 46.8% (15/32) and ciprofloxacin 43.75% (14/32). However, most of the antibiotics that are active against GNB like ticarcillin-clavulanic acid, piperacillin-tazobactum, cefoperazone, cefepime, aztreonam, meropenem, imipenem, amikacin and colistin were found to be resistant to E. meningoseptica. Similar antibiotic profile was observed in study done by Joo HD et al., in Korea, where 90% of isolates were susceptible to minocycline and fluoroquinolones, including levofloxacin (66.7%) and ciprofloxacin (60%) [16]. There are also other studies that have shown isolates that were susceptible to amikacin, piperacillin-tazobactam, trimethoprim/sulfamethoxazole, ciprofloxacin, levofloxacin, and gentamicin [6]. Han MS et al., studied the prevalence and antibiotic susceptibility of E. meningoseptica by using 16s RNA sequencing and found that isolates were 100% and 94% susceptible to piperacillin-tazobactam and rifampin, respectively, but only 23% to 41% were susceptible to fluoroquinolones [13]. Zong Z had reported a very high degree of resistance to fluroquinolones and piperacillin-tazobactum in E. meningoseptica isolates from bile samples [17]. These differences in resistance patterns could be due to lack of standard guidelines for performing antibiotic susceptibility. Currently, CLSI does not provide any validated susceptibility testing method or MIC break points for E. meningoseptica [8,18]. The antibiotic sensitivity pattern depends on the species of Elizabethkingia, source of the isolate and time of bacterial isolation [13].
There are no treatment guidelines regarding the choice of antibiotic for E. meningoseptica. The varying susceptibility patterns make it essential for keeping a track of the resistance patterns of the organism. Though it was considered that vancomycin has been the drug of choice for treating E. meningoseptica, high MIC of >16 mg/dL and discrepant results between disc diffusion and broth micro dilution for vancomycin using Staphylococcus aureus break points, has made it a difficult option to choose from [7,9]. Vancomycin is used only as reserve drug in our setting based on the hospital antibiotic policy hence the use of the same was restricted to only those patients in whom other drugs were resistant.
The use of combination therapy rather than use of monotherapy for successful treatment of the patients is recommended [7]. Although the in-vitro activity of newer fluoroquinolones, minocycline, tigecycline, and rifampicin is good, it has been shown that E. meningoseptica infections respond well when antibiotic combinations are given. These include piperacillin-tazobactam plus rifampicin, vancomycin plus rifampicin, or a fluoroquinolone combined with vancomycin and rifampicin [7].
Though in the current study the mortality rate was high (45%) it could not be attributed to the organism as such because of the co-existing comorbidities of the patients. However, many studies have shown varying range of mortality rate i.e. from 23% to 75% [6,11]. Such high rates of mortality could be due to the resistance of the organism to the common gram negative antibiotics, difficulty and delay in identification of the organism leading to incomplete antibiotic coverage [2]. A Taiwanese’s study showed that their 14 day mortality attributed to E. meningoseptica was significant, though antibiotic reports of the organism were available only 12% of their study population had received the appropriate drug as most of the clinicians thought that E. meningoseptica could only be a coloniser or a contaminant [19].
Limitation
Small sample size, inability to attribute the pathogenicity of the organism to patient mortality and lack of proper control group are the major concerns of this study.
Conclusion
Nosocomial infections caused by emerging pathogens like E. meningoseptica are an increasing problem in healthcare settings. It is likely to be encountered more frequently in the future because of its endurance in environment and resistance to multiple antibiotics. It is, therefore, essential that laboratories have the ability to identify the organism correctly and also perform antibiotic sensitivity testing. There are very few studies of E. meningoseptica infections in patients with liver and biliary diseases, especially from India. These patients with E. meningoseptica infection have significant morbidity and mortality. Hence awareness about the organism, active surveillance and investigation for the source of the organism will help in implementation of appropriate infection control measures and prevent outbreaks or recurrence of E. meningoseptica infections.