In India, head and neck cancers are the leading cause of cancer morbidity and mortality with an age adjusted incidence in males of 10.8 to 38.8 per 100,000 males and 6.4 to 14.9 per 100,000 females. One of the standards of care in the management of advanced HNSCC, is radiotherapy and chemotherapy used concurrently [1-3]. Although there is a benefit [3] obtained by these chemotherapeutic agents, the toxicity and cost of the total treatment have increased significantly.
Thus, despite encouraging clinical progress, there is still a clear need for further improvement of the therapeutic outcome in locally advanced HNSCC. Altered fractionation schedules have been one such area of intensive research. A variety of fractionation schedules including standard fractionation, hyper fractionation, accelerated fractionation and their variants have been used in radiotherapy of HNSCC [4-7]. Accelerated fractionation is based on the rationale that a reduction in Overall Treatment Time (OTT) decreases the opportunity for tumour cells to regenerate during treatment [8]. Nguyen LN and Ang KK, in their review article of fractionation for head and neck cancer, reviewed 18 trials of altered fractionations including over 6000 patients and gave a general conclusion which can contribute to improving the standard of care for patients with HNSCC that “a modest acceleration of radiotherapy by one week without total dose reduction or a break in the treatment, by giving six fractions of 2 Gray per week, consistently yields better locoregional control of head and neck carcinomas without much increase in late toxic effects” [9]. However, it was also concluded that the effect on survival remains to be determined from future follow-up data.
A promising report has come from the Danish group in a randomised controlled trial [10]. It has been concluded saying that the shortening of OTT by the increase of the weekly number of fractions is beneficial in patients with HNSCC. After the success of this trial, six-fractions-weekly regimen has become the standard treatment in Denmark. It seems that just by adding one more fraction of radiotherapy, weekly, better locoregional control can be achieved with marginal increase in toxicity.
At the time of conception of this trial no randomised trial had been reported comparing six fractions a week radiotherapy regimen with concurrent chemoradiotherapy in HNSCC. The hypothesis was that if the magnitude of benefit is equivocal between both these treatment regimens then six fractions a week regimen would be more cost-effective. Reducing treatment time by one week would not only have radiobiological impact on tumour control but also would be of great help to those poor patients who had to come from long distances and had to manage their stay during the period of radiotherapy treatment.
This was a non-inferiority study that aimed to compare six fractions a week radiotherapy regimen with concurrent chemoradiotherapy and to determine if accelerated fractionation can serve as an acceptable substitute for the current standard; that is CTRT in locally advanced HNSCC.
Materials and Methods
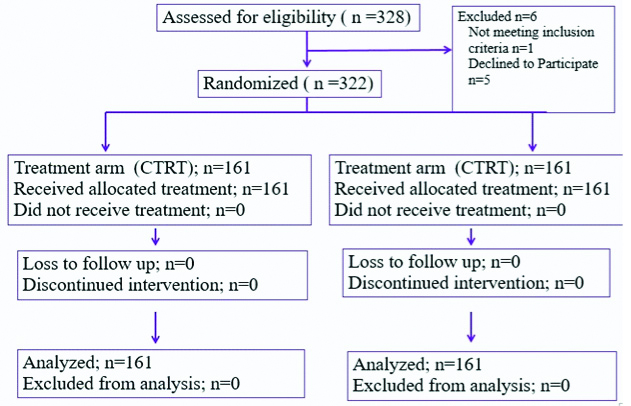
This is a prospective randomised controlled trial conducted at the Department of Radiation Oncology, King George’s Medical University, Lucknow. 328 eligible patients were randomly assigned to five fractions per week of 2 Gray along with concurrent chemotherapy (CTRT Arm) or to six fractions per week of 2 Gray each (AFRT Arm) between March 2006 and March 2008. Randomization was done by computer generated random numbers after the patients met eligibility criteria. Eligible patients were biopsy proven HNSCC with sub-sites including glottic, supraglottic, oropharynx and hypopharynx (excluding nasopharynx and oral cavity), stage III to IV (AJCC 2002, 6th edition), age: 20-70 years, Karnofsky performance score more than or equal to 70, adequate bone marrow, hepatic, and renal functions, white blood cell count >3500/mm3, hemoglobin >10g/dL, platelet count >100,000/mm3, total bilirubin <1.5 mg/dL, liver transaminase <2 times upper limit of normal, serum creatinine <1.5 mg/dL. Criteria for ineligibility included severe concomitant illness such as uncontrolled cardiovascular disease, uncontrolled diabetes mellitus, uncontrolled psychological disorders, active infection, active synchronous cancer, prior radiotherapy or chemotherapy, history of other malignancies within the past 5 years except basal cell carcinoma and squamous cell carcinoma in-situ of the skin and cervical cancer in-situ and patients who are pregnant or lactating. Patients underwent a full clinical examination before treatment, together with recording of histopathology and differentiation of the primary tumour, Tumour, Node, Metastasis (TNM) classification; assessment of the localization and the size of the primary tumour and regional lymph-node metastases by either clinical examination, endoscopy, radiography, CT scan, assessment of performance status according to WHO criteria; and chest radiograph.
Of 328 recruited patients, six patients were found to be ineligible and were excluded from the study.
The trial was done in accordance with the Declaration of Helsinki and approved by Institutional Ethical Committee [Ref No. 13 (8127-A)/2006]. Informed consent was obtained from all participants.
Radiotherapy Procedures
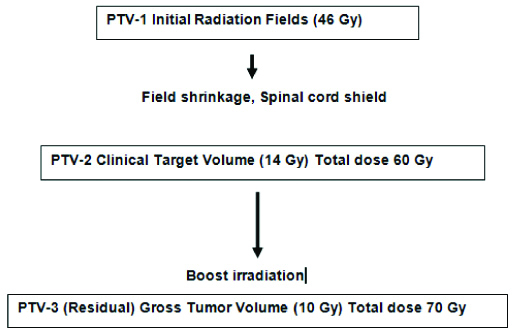
Patients were treated with external radiotherapy given with either a linear accelerator or Cobalt-60 Machine (Theratron, 780-C) The treatment principles and dose specifications agreed with the guidelines given in the ICRU-50/62 report. The radiotherapy protocol was as described in the [Table/Fig-1]. The dose to primary tumour and neck nodes for both arms was as follows: primary tumour 70 Gray, neck nodes if N0: 46-60 Gray, node positive neck: 66-70 Gray, spinal cord Dmax <45-50 Gray. No planned neck dissection was done before or after radiation. CTRT arm received chemotherapy as Cisplatin 35-40 mg/m2/week for a total of 7 cycles along with radiotherapy. In cases of residual tumour, recurrence, or progression of the disease, salvage surgery was done, or palliative treatment given, depending on the status of the individual patient, symptoms, and previous treatment.
Radiotherapy protocol of the study.
Statistical Analysis and End Points
Assuming a hazard ratio of 0.652 in favour of control group and assuming alpha=0.05, power of the study (probability of correctly detecting the real effect) as 80%, 286 patients had to be recruited (assuming equal allocation, 143 in each arm). Also, with the assumption of attrition rate of 10-15%, the sample size to be recruited had to be between 315-328. Hence, 322 patients were recruited 161 in each arm.
The primary end point was five-year Loco Regional Control (LRC) defined as complete and persistent disappearance of the disease in the primary tumour and regional lymph nodes after radiotherapy, not including salvage procedures. Secondary endpoints included disease specific survival (DFS), Overall Survival (OS), and early and late treatment-related morbidity and compliance rate. Acute and late radiation morbidities were graded as per Radiation Therapy Oncology Group (RTOG) morbidity scoring criteria [11-13]. Failure was recorded in the event of recurrence, or if the primary tumour never completely disappeared, in which case the tumour was then assumed to have failed at the time of randomization. All time estimates were calculated using the date of randomization as the initial value. We planned to follow-up patients for at least 5 years or until death.
The actuarial values of the endpoints were assessed by the Kaplan-Meier product-limit method using the SPSS (version 17.0). The p- values estimated were for a two-tailed test, and the significance level was set at 5%.
Results
Between March 2006 and March 2008, 328 patients were recruited and randomly assigned to treatment. Out of these, six patients were found to be ineligible and were excluded from the study [Table/Fig-2]. The baseline characteristics of the 322 evaluable patients are shown in [Table/Fig-3]. There were 292 men and 30 women, with a median age at Randomisation of 52 years (range 30-77 years); there were no significant differences in terms of tumour characteristics between the groups. Compliance with radiotherapy was good in both treatment groups, with (90%) patients assigned to receive CTRT and (95%) of those assigned to receive AFRT completing planned radiotherapy as per protocol.
Trial profile according to CONSORT recommendations.
Baseline characteristics of patients according to treatment group.
| Variables | Five fractions per week and concurrent chemotheraphy (N=161) | Six fractions per week (N=161) |
|---|
| Age (years) |
| <55 | 119 | 88 |
| 55-56 | 33 | 49 |
| >65 | 9 | 24 |
| Sex |
| Male | 145 | 147 |
| Female | 16 | 14 |
| WHO PS |
| <70 | 21 | 12 |
| 70-80 | 79 | 115 |
| >80 | 61 | 34 |
| Site |
| Oropharynx | 74 | 75 |
| Larynxor hypopharynx | 87 | 86 |
| T stage |
| T1-T2 | 11 | 12 |
| T3-T4 | 150 | 149 |
| Nodal status |
| Negative | 48 | 47 |
| positive | 113 | 114 |
| Stage |
| Stage 3 | 38 | 21 |
| Stage 4 | 123 | 140 |
| Differentition |
| Well | 55 | 56 |
| Moderate | 46 | 44 |
| Poor | 60 | 61 |
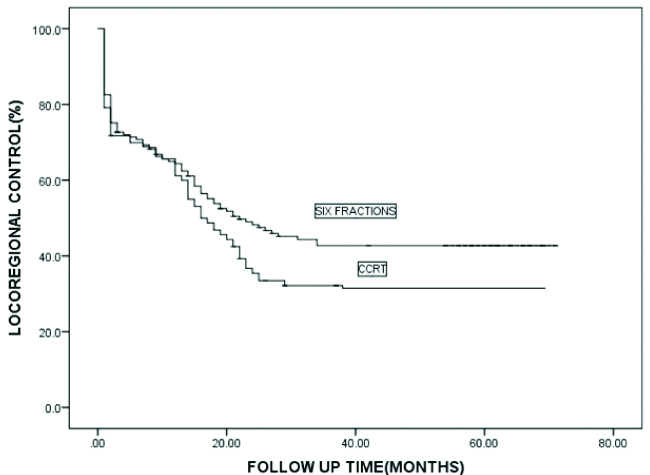
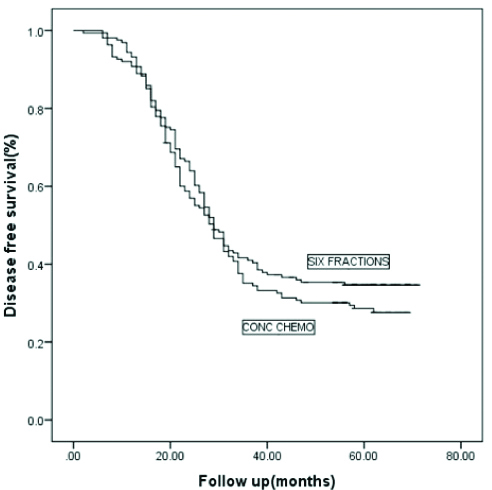
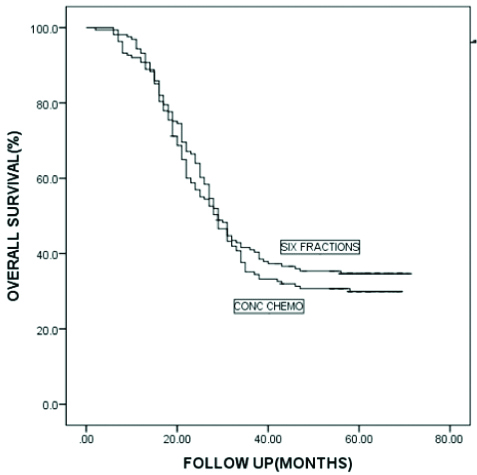
Median OTT was 43 days for the AFRT arm and 49 days for the CTRT arm. At the time of final assessment, after a mean follow-up since Randomisation of 35 months (range 6-71 months), 198 patients had failed to achieve persistent locoregional control after primary irradiation. 169 patients died from HNSCC, with 171 deaths overall. At 5 years, the actuarial rate of locoregional control [Table/Fig-4] was slightly worse for patients in the CTRT group than for those in the AFRT group (32% vs. 42% at 5 years), this difference was of borderline statistically significant; (p=0.071). At 5 years, the actuarial rate of DFS [Table/Fig-5] was 28% for those patients in the CTRT group versus 35% for those in the AFRT group with this difference not statistically significant (p=0.282). The OS [Table/Fig-6] was 30% for those in the CTRT group versus 35% for those in the accelerated group (p=0.388).
Kaplan Meier analysis of locoregional control for two arms.
Kaplan Meier analysis of disease-free survival for two arms.
Kaplan Meier analysis of overall survival for two arms.
Regarding the acute toxicity analysis, while Grade II mucositis was more common in the AFRT arm, confluent mucositis was more common in the CTRT arm and these differences were statistically significant. The more frequent severe mucosal reactions in the CTRT group resulted in a significantly increased incidence of Ryle’s tube placement during treatment compared with the AFRT group with a longer median duration of placement. Acute severe skin reactions were not significantly different in the AFRT arm than in the CTRT arm [Table/Fig-7]. However, with regards to the late reactions, there was a trend to significance for both Grade 3 skin as well as subcutaneous toxicity in the AFRT arm whereas the increased incidence of Grade 3 reactions in the CTRT arm did not translate into more severe late mucosal sequelae. The present authors also found that the incidence of late Grade 2-3 xerostomia was more in the CTRT arm [Table/Fig-8].
Acute radiation morbidity between treatment arms.
| Variables | AFRT arm | CCRT arm | p-value |
|---|
| Ryle’s tube | 0.001 |
| Required | 17 | 62 |
| Not required | 124 | 80 |
| Required but | | |
| Refused | 20 | 19 |
| Skin toxicity | 0.315 |
| Grade 1 | 26 | 15 |
| Grade 2 | 99 | 108 |
| Grade 3 | 35 | 39 |
| Grade 4 | 1 | 1 |
| Mucositis | 0.015 |
| Grade 1 | 4 | 8 |
| Grade 2 | 122 | 100 |
| Grade 3 | 34 | 55 |
| Duration of Ryle’s tube (days) | 45 | 50 | 0.001 |
Late radiation-related morbidity by fractionation schedule.
| Variable | CCRT | Six fractions | p-value |
|---|
| Skin toxicity | 0.053 |
| Grade 1 | 41 | 41 |
| Grade 2 | 112 | 96 |
| Grade 3 | 5 | 16 |
| Grade 4 | 5 | 8 |
| Mucositis | 0.968 |
| Grade 1 | 92 | 90 |
| Grade 2 | 60 | 61 |
| Grade 3 | 11 | 10 |
| Subcutaneous toxicity | 0.051 |
| Grade 1 | 38 | 34 |
| Grade 2 | 115 | 102 |
| Grade 3 | 10 | 24 |
| Grade 4 | 0 | 1 |
| Salivary | 0.042 |
| Grade 1 | 5 | 16 |
| Grade 2 | 94 | 88 |
| Grade 3 | 64 | 57 |
Discussion
The benefit for CTRT vis-à-vis radiotherapy alone has been demonstrated in several meta-analyses [14]. Altered fractionation is another treatment approach that has improved the poor results of conventional radiotherapy in this group of patients especially hyper-fractionation and accelerated radiotherapy. The Meta-analysis of Radiotherapy in Carcinoma of Head and Neck (MARCH), of more than 6500 HNSCC patients, has confirmed a survival benefit of altered fractionation [15]. In the MARCH analysis, altered fractionated demonstrated a 23% reduction in local failure, at five years, as well as a benefit in LRC (13% reduction in the risk at five years). The present study was a randomised comparison of the efficacy and toxicity of the two regimens; the DAHANCA protocol [10] of AFRT given as 6 fractions a week and the current standard in head and neck cancer of CTRT.
The 5-year OS and LRC of present patient cohort was 32% and 37% respectively. This agrees with international data which puts the OS of locally advanced HNSCC between 30-50% [16-24]. The predominance of T4 patients (73%) and Stage 4 patients in the study (82%) might explain the present survival results being at the lower end of this spectrum.
The predominant pattern of failure in patients in the present study was locoregional with nearly 40% of the patients developing a local or a regional recurrence or both with only 8% developing distant metastasis. This is in accordance with published data which show locoregional as being the predominant mechanism of failure in these patients [14-24]. The rate of distant metastasis is also within the range of 5-10% cited by most studies. Owing to this, there was a strong correlation between the locoregional control and disease specific survival and as such both were similar for the two trial arms. Of note, while the number of local or regional recurrences were similar in the two arms (34 vs. 33), the number of distant metastasis were three times in the accelerated arm than in the concurrent chemoradiation arm (20 vs. 6) and this difference was statistically significant (p=0.004). This, however, did not impact the overall survival and there are perhaps certain implications of this observation. It is debated whether weekly concurrent chemotherapy impacts distant metastasis at all [14,25,26], and if so, is it by impacting locoregional failure or by acting on micro-metastatic disease or both. The available data is controversial regarding equivalent efficacy even if provided a certain cumulative dose of weekly cisplatin is given with varying toxicity results [27,28], However, weekly cisplatin continues to be used in most Indian institutions as the preferred regimen [29]. Based on the present study results, while the locoregional control was similar for the two arms, the rate of distant metastasis was not, implying that the latter mechanism may be the reason for these findings. However, it must be noted that the nodal failures were slightly higher in the altered fractionation arm and although not statistically significant, nodal metastasis is often a harbinger of distant metastasis.
Compliance with radiotherapy was good overall but was different in both treatment groups, with 95% (153/161) patients assigned to receive six fractions and 147/163 (91%) of those assigned to receive five fractions receiving their planned radiotherapy dose. Although this difference was not statistically significant, the present authors do acknowledge that it could have impacted our results and led to inferior outcomes for the CTRT arm. The compliance rates were much better than the TMH trial [18] (82%) and almost as good as the DAHANCA trial [10] (98%). This might be the reason for the positive results of the DAHANCA trial vis a vis the negative results of the TMH trial.
The median OTT in accelerated RT arm was 43 days (37-78) and in the chemoradiation arm was 49 days (41-65). The treatment time in the accelerated arm compares well with the RTOG trial [22], the IAEA-ACC trial [23] and is slightly higher vis a vis the DAHANCA trial [10]. The reduction in OTT by 1-week improved LRC (70 vs. 60%), DFS (73% vs. 66%) but not OS in the DAHANCA-7 trial. The lack of improvement in overall survival is not a sine qua non of efficacy as the poor general condition of these patients often leads to them having competing causes of mortality [24].
Looking at the present toxicity results, while acute grade II mucositis was more common in the accelerated arm, confluent mucositis was more common in the CTRT arm and these differences were statistically significant. The overall rate of Grade 3 mucosal reactions in the six fractions arm (21%) and CTRT arm (35%) were well within the rates of 28-77% reported by most investigators [10,16-23]. The increased incidence of Grade 3 mucosal reactions in our chemoradiation cohort is in accordance with other studies that have used the same accelerated schedule [18,19]. There was an increased incidence of Grade 2-3 xerostomia in the chemoradiation arm in our trial as well as the above-mentioned studies and while these differences were not statistically significant, they concur with the meta-analysis [22] that suggests that AFRT reduces late xerostomia vis a vis CTRT. Interestingly, while the acute skin toxicity was not significantly different between the two arms there was a trend for worse late skin Grade 3 toxicity in the altered fractionation arm. One possible reason for this could be that the acute reactions in this arm showed delayed healing vis a vis the CTRT arm. Although such reactions have not been seen in other recent trials of AFRT [10,18,23], the incidence of such reactions is multifactorial [30-32] and will be investigated further.
We did not do HPV testing for our oropharyngeal cancer patients as this was not standard at the time the trial was conceived. The incidence rates of HPV in Indian patients is not known at present although single institutional studies exist and estimate the rate at about 22% in a predominantly north Indian population [33].
This is less than our western counterparts and although ours was a predominantly north Indian population, considering the present patient demographic with only 8% patients less than 40 and mostly heavy smokers and alcoholics we assume that the number would have been lower. HPV as a prognostic factor in the Indian context is still debatable [34]. Lastly, the impact of altered fractionation is not influenced by HPV positivity or negativity as has been shown by the RTOG 0129 trial [35].
Thus, while incorporation of altered fractionation in head and neck management paradigms as a substitute for CTRT is perhaps premature, is there perhaps a subgroup of patients that can be managed with altered fractionation alone at present? This is not a new concept and has been suggested by earlier authors [36,37] but is not widely followed in the oncology community. It is recommended that patients of unfavourable T2 or exophytic T3 with N0- N1 disease constitute an intermediate risk subgroup and should be considered for altered fractionation alone given that in these patients, it achieves a good balance of improving locoregional control and at the same time maintaining an acceptable toxicity profile. Even a stage based indirect comparison of the effects of chemoradiation reveals a greater benefit of CTRT in Stage 4 versus Stage 3 in the MACH NC metanalysis [14]. Finally, even the MARCH metanalysis [15] suggests that altered fractionation should be considered for N0-N1 disease with chemoradiation reserved for more advanced nodal disease. Thus, altered fractionation could be considered a good option for these patients particularly in the Indian subcontinent based on available evidence and our study.
The strengths of the present study were that it had a prospective randomised design, achieved its planned accrual, had a good sample size and had a median follow-up of 30 months.
Limitation
The limitations include non-usage of intensity modulated radiotherapy, lack of routine HPV testing and lack of quality of life assessment which would have allowed us to further define the magnitude or lack of benefit of AFRT over CTRT.
Conclusion
Accelerated Fractionated Radiotherapy yields comparable and descent clinical outcomes as compared to concurrent chemoradiotherapy in head and neck squamous cell carcinoma. This also has better tolerability and safety profile and would be particularly valuable in intermediate risk group (T2-T3, N0-1) patients.