Breast Cancer (BC) is the most frequently diagnosed female cancer worldwide and is a leading cause of cancer-related deaths in women [1]. In Egypt, BC accounts for 38% of all female cancers according to the National Cancer Institute, Cairo University [2]. Currently, the prognosis and treatment of BC are dependent on tumour grade and stage, as well as 3 main protein markers: Oestrogen Receptor (ER), Progesterone Receptor (PR) and Human Epidermal Growth Factor Receptor 2 (HER2) [3]. To the best of authors knowledge, no immunotherapy is currently widely used, for treatment of BC, as BC hasn’t been generally considered an immunogenic cancer. However, some investigators have begun to consider novel immunotherapeutic strategies for treatment of BC [4].
The immune system recognises cancer cells as soon as they emerge. The interaction between T-cells and Antigen-presenting cells (APC) is complex and involves the T-cell receptor as well as, multiple co-regulatory receptors, which exert either activating or inhibitory stimuli to the T-cells [5]. Tumours can resist immune attack by expressing ligands that engage inhibitory receptors and dampen T-cell functions [6].
The programmed death pathway is one of the major immune response checkpoint and a target for cancer immunotherapy [7]. PD-1 is a transmembrane protein and member of the B7-CD28 family of co-regulatory molecules expressed by activated lymphocytes [8,9]. It has two known ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC) [6]. The interaction between PD-1 and its ligands inhibits T-cells, blocking immune responses [10].
Several studies investigated PD-L1 expression in BC. They showed variable rates of expression within the TC and Tumour Infiltrating Lymphocytes (TILs). The literature also showed contradictory results about the prognostic value of PD-L1 expression in BC. While some confirmed PD-L1 expression in BC as a marker of poor prognosis [27], others showed association of PD-L1 expression in BC with more favourable prognosis [9,28].
This study aimed at Investigation of immunohistochemical expression of PD-L1 in TC and TILs of female breast carcinoma, as well as, correlation of this expression with the BC subtypes, extent of tumour infiltrating lymphocytes and other available pathological aspects of the tumour, including tumour histological type, grade and stage.
Materials and Methods
This study was a retrospective observational cross-sectional study. A total of 100 formalin fixed, paraffin embedded full-face BC tumour tissue sections were collected from the archive of the Pathology Department at the Kasr El Aini Hospital (Cairo university hospital), in the time period between March 2015 and October 2017. The authors obtained the approval of Ethical Committee in the faculty of Medicine, Cairo University (N-115-2016).
Inclusion criteria included cases diagnosed as BC subjected to radical or conservative mastectomy with axillary dissection and has performed immunohistochemical evaluation for ER, PR, HER2 and Ki-67.
Exclusion criteria included: 1) Cases with missing data or no available IHC report; 2) Cases who received neo-adjuvant therapy; either hormonal or chemotherapy; 3) Patients subjected to lumpectomy or simple mastectomy without axillary sampllng; 4) Cases with HER2 Score 2 by IHC, with no available SISH/DISH report.
The data obtained from the pathology reports of the cases included age of patient, tumour size and Lymph Node (LN) status. An IHC report including the ER, PR, HER2 and Ki67 results was obtained for each case. A Dual In Situ Hybridization (DISH) report was obtained for cases with HER2 class 2+.
Haematoxylin and eosin stained sections were prepared for histopathological examination. The specimens were anonymous for confidentiality and replaced by numbers. The tumours were histologically typed according to the latest available World Health Organisation classification (WHO 2012) [29]. Histological grading was performed according to the Nottingham Grading System [30]. For further statistical evaluation, Grade 1 and 2 cases were considered as low grade, while Grade 3 cases were considered as high grade [31, 32]. Regarding lobular carcinomas, classic lobular carcinoma cases were considered as low grade and pleomorphic lobular carcinoma cases were considered as high grade [33]. Lympho-vascular invasion (LVI) was defined as presence of tumour cells within an endothelial lined space (lymphatic and/or blood vessel) outside the border of the tumour [34]. The slides were also examined for the presence of tumoural necrosis.
Regarding the TILs, they were scored following the recommendations of the International TILs Working Group 2014. Briefly, all stromal mononuclear cells within the borders of the invasive tumour were evaluated and reported as a percentage value of the stromal area (i.e., percentage of the stromal area occupied by mononuclear inflammatory cells) and not as a percentage of the stromal cells. TILs outside of the tumour border, around DCIS, ADH and normal breast tissue, as well as in areas of necrosis and hyalinosis, if any, were not included in the scoring. The working group recommended full assessment of average TILs in the examined tumour area; they don’t recommend focusing on ‘hot spots’ [35]. As the working group didn’t recommend a clinically relevant threshold[s] for TILs assessment [36], the present authors scored stromal TILs subjectively in 10% increments. Tumours were classified as high TILs (≥30%) or low TILs (<30%) [11,36].
Tumours staging was performed using the TNM staging system. The cases were further divided into anatomic stages and prognostic stages according to the 8th edition of the AJCC staging manual [37]. Regarding the prognostic stages, 15 cases were excluded as their prognostic stages were missing in the AJCC classification. The stages (whether anatomic or prognostic) were further subdivided into early; Stages I and II and advanced; stages III and IV, for statistical purposes [38].
Regarding the BC subtyping, the tumours were classified according to the St. Gallen International Expert Consensus 2013 recommendations, which stated that although identification of intrinsic subtypes is more accurate using molecular techniques, surrogate definitions of BC subtype can be obtained by IHC evaluation of ER, PgR, Ki-67 and HER2, in countries where routine molecular studies is not available for all cases [39]. Luminal cases having high histological grade were considered as luminal B rather than luminal A, regardless of their Ki-67 index, according to St. Gallen International Expert Consensus 2017 recommendations [40]. This subtyping was also supported by the 8th edition of the AJCC staging manual released in 2017 [37].
Paraffin sections were cut at 4 μm thickness on positively charged slides and stained manually for immunohistochemistry in the tumour marker unit of the Pathology Department at Kasr El Aini Hospital. The sections were deparaffinized in xylene, dehydrated in ethanol, then washed in Tris-buffer Physiological Saline (TBS). Antigen retrieval was performed by heating the sections in sodium citrate buffer. The sections were then washed in TBS buffer. Endogenous peroxide blocking was then achieved by 0.3% hydrogen peroxide in methanol, followed by washing in TBS for 15 minutes.
The primary antibody was anti-PD-L1 antibody (rabbit polyclonal, 0.2 mL concentrated, obtained from Novus Biologicals). The antibody was diluted with TBS, pH 7.4. The sections were incubated overnight with the diluted antibody. The sections were then incubated for 30 minutes with the secondary antibody (4.5 μL biotinylated anti-mouse antibody in 1 mL of 1% BSA), then washed in TBS buffer. The slides were finally stained with Diaminobenzidine tetrahydrochloride (DAB) solution, then in distilled water. Hematoxylin was used as a counterstain.
PD-L1 immunohistochemical staining was scored in the tumour cells and the stromal TILs separately:
Regarding the TC PD-L1 expression, although both cytoplasmic and membranous positivity was observed as reported by many other authors [11,23,41-44], only membranous staining was considered for further analysis [45] as PD-L1 localisation to the cell membrane is likely required for interaction with PD-1 [46].
Cases were considered as positive if 1% of tumour cells stained for PD-L1 with any staining intensity. This threshold was selected based on data demonstrating clinical response to PD-L1 inhibition at this expression level in some cancers. Further scoring was performed as follows: Score 1; 1% to 5%, Score 2; 6% to 10%, Score 3; 11% to 25%, Score 4; 26% to 50% and Score 5; >50% positive tumour cells [47].
Tumour infiltrating lymphocytes staining was considered as positive when >5% of stromal TILs showed PD-L1 reactivity with any intensity and was further scored as follows: Score 1; 5% to 10%, Score 2; 11% to 25%, Score 3; 26% to 50% and Score 4; >50% positive TILs [47].
Statistical Analysis
The previously mentioned histopathological and immuno-histochemical data were then transferred to the SPSS Software program, version 25.0 to be statistically analysed. Data were summarised using frequency and percentages. Comparison between groups was then performed using chi-square test. Statistically significant results were considered if p-value was ≤0.05.
Results
This study included 100 cases of BC obtained from modified radical mastectomy and conservative breast surgery specimens. The pathological data of the cases are summarised in [Table/Fig-1].
The pathological data of the collected cases.
| Parameter | | Number (%) |
|---|
| Histological type | IC-NST | 76 (76%) |
| ILC | 10 (10%) |
| Carcinoma with medullary features | 7 (7%) |
| Mixed duct and lobular | 2 (2%) |
| Mucinous carcinoma | 2 (2%) |
| Encysted papillary carcinoma with micro-invasion | 1 (1%) |
| Tubular carcinoma | 1 (1%) |
| Cribriform carcinoma | 1 (1%) |
| Histological grade | Low | 57 (57%) |
| High | 43 (43%) |
| Tumoural necrosis | Positive | 29 (29%) |
| Negative | 71 (71%) |
| T stage | T1 | 10 (10%) |
| T2 | 75 (75%) |
| T3 | 13 (13%) |
| T4 | 2 (2%) |
| N stage | 0 | 45 (45%) |
| 1 | 26 (26%) |
| 2 | 17 (17%) |
| 3 | 12 (12%) |
| Anatomic stage | Early (I and II) | 66 (66%) |
| Late (III and IV) | 34 (34%) |
| Prognostic stage | Early (I and II) | 47 (55.3%) |
| Late (III and IV) | 38 (44.7%) |
| ER | Positive | 74 (74%) |
| Negative | 26 (26%) |
| PR | Positive | 70 (70%) |
| Negative | 30 (30%) |
| Her2-neu | Positive | 15 (15%) |
| Negative | 85 (85%) |
| Ki-67 index | Low | 50 (50%) |
| High | 50 (50%) |
| BC subtypes | Luminal A | 32 (32%) |
| Luminal B | 42 (42%) |
| HER2 enriched | 10 (10%) |
| Triple negative | 16 (16%) |
| LVI | Positive | 49 (49%) |
| Negative | 51 (51%) |
| Stromal TILs | Low | 78 (78%) |
| High | 22 (22%) |
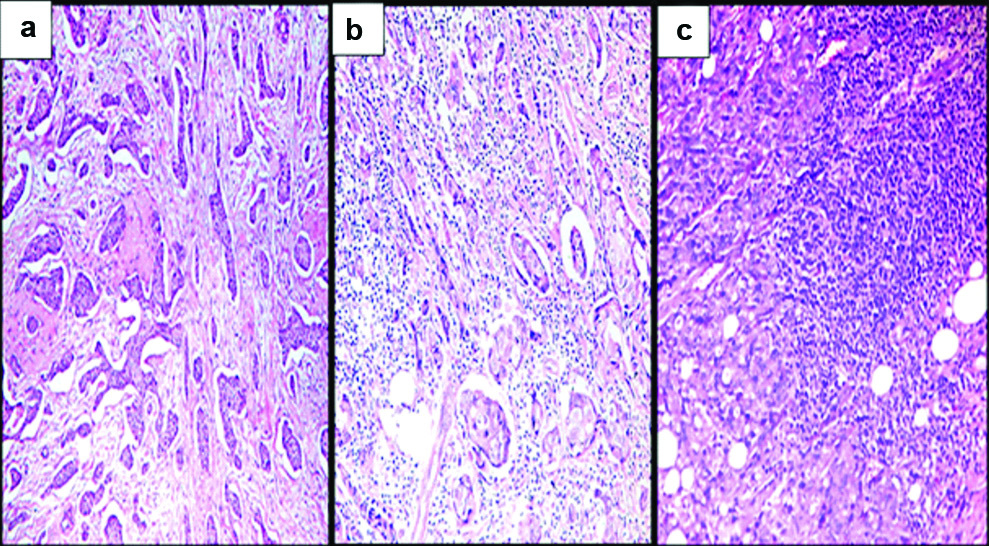
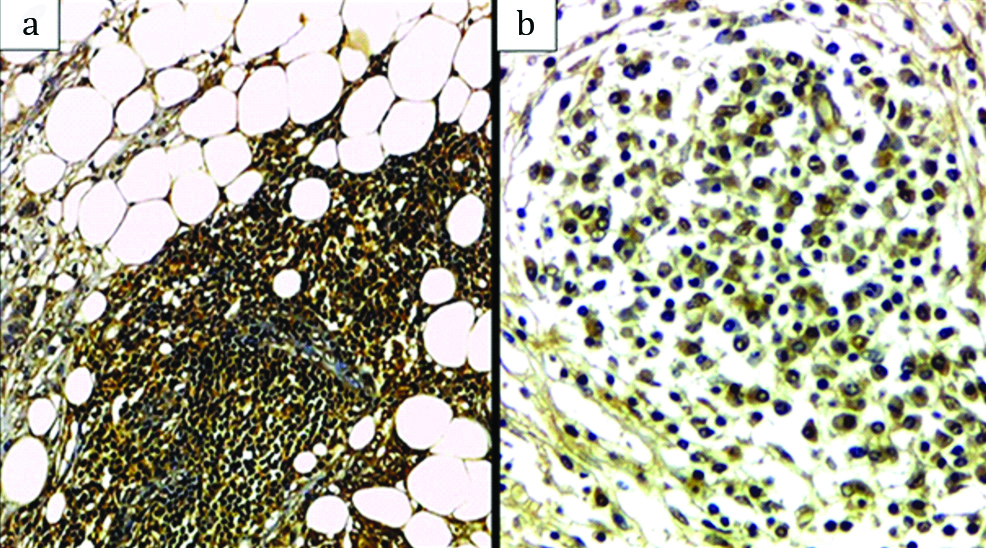
Regarding the extent of stromal TILs, 78 cases showed low TILs (78%) [Table/Fig-2a] and 22 cases showed high TILs (22%) [Table/Fig-2b,c].
H&E stained sections of the studied cases: a) Low stromal TILs IC-NST (<30% of the stroma) (x100); b) High stromal TILs IC-NST (≥30% of the stroma) (x100); c) High stromal TILs carcinoma with medullary features (≥30% of the stroma) (x100).
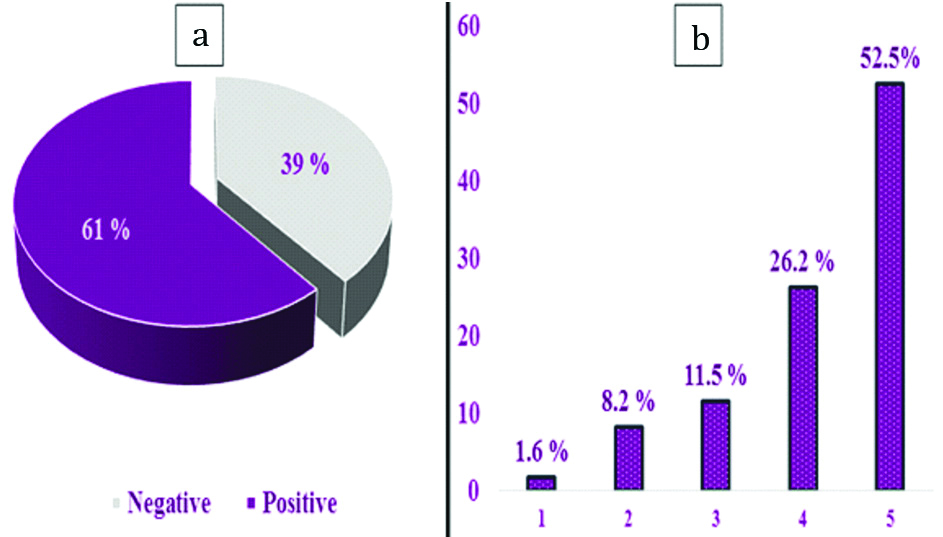
As regards TC PD-L1 expression, it was positive in 61 cases (61%) and negative in 39 cases (39%). Among the positive cases, 1 case (1.6%) was Score 1, 5 cases (8.2%) were Score 2, 7 cases (11.5%) were Score 3, 16 cases (26.2%) were Score 4 and 32 cases (52.5%) were Score 5 [Table/Fig-3a,b,4a,b].
Histogram showing Expression and scores of TC PD-L1: a) TC PD-L1. Expression; b) Positive TC PD-L1 cases scores.
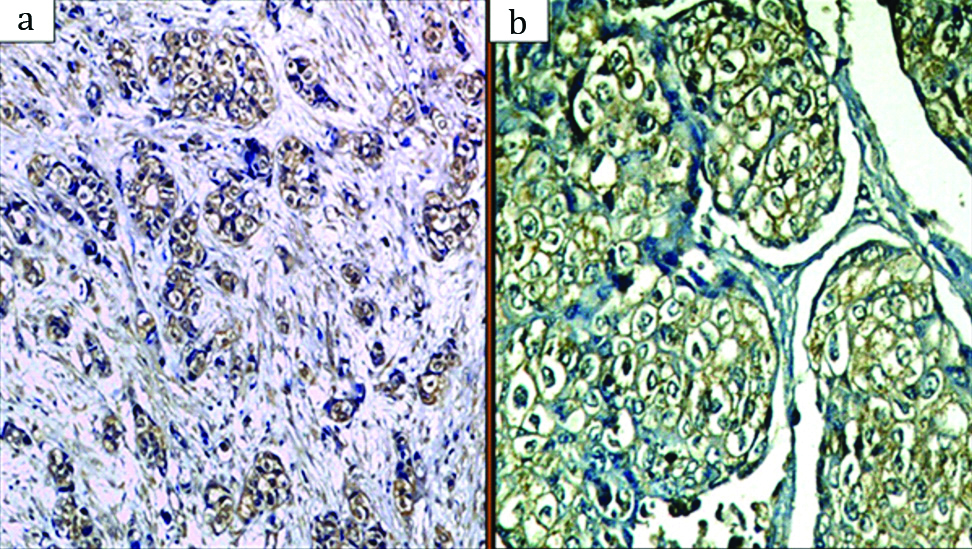
Immunohistochemical expression of PD-L1 in TC showing mainly membranous staining: a) (×100); b) (×200).
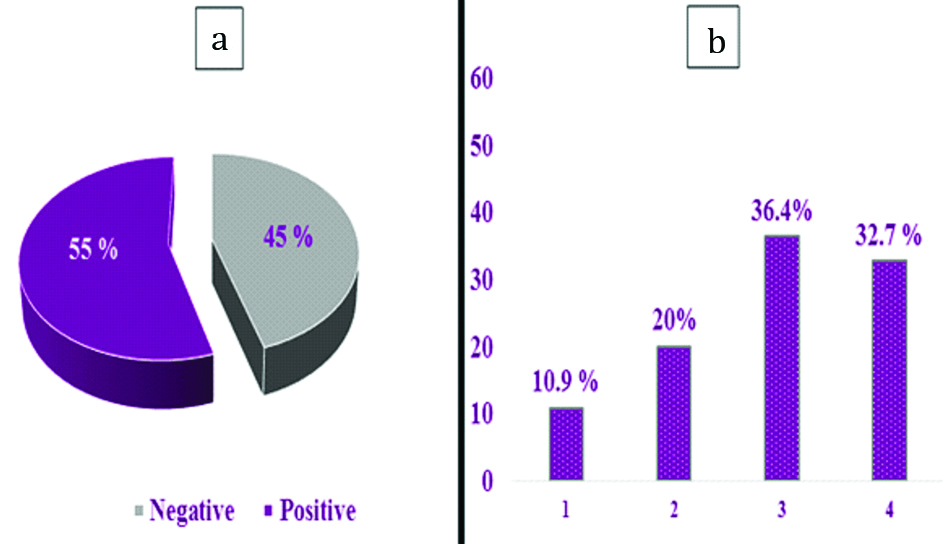
Stromal TILs PD-L1 expression was positive in 55 cases (55%) and negative in 45 cases (45%). Among the positive cases, 6 case (10.9%) were Score 1, 11 cases (20%) were Score 2, 20 cases (36.4%) were Score 3 and 18 cases (32.7%) were Score 4 [Table/Fig-5a,b,6a,b].
Histogram showing Expression and scores of TILs PD-L1: a) TILs PD-L1 expression; b) Positive TILs PD-L1 cases scores.
Immunohistochemistry expressions of PD-L1 in TILs: a) (×200); b) (×400).
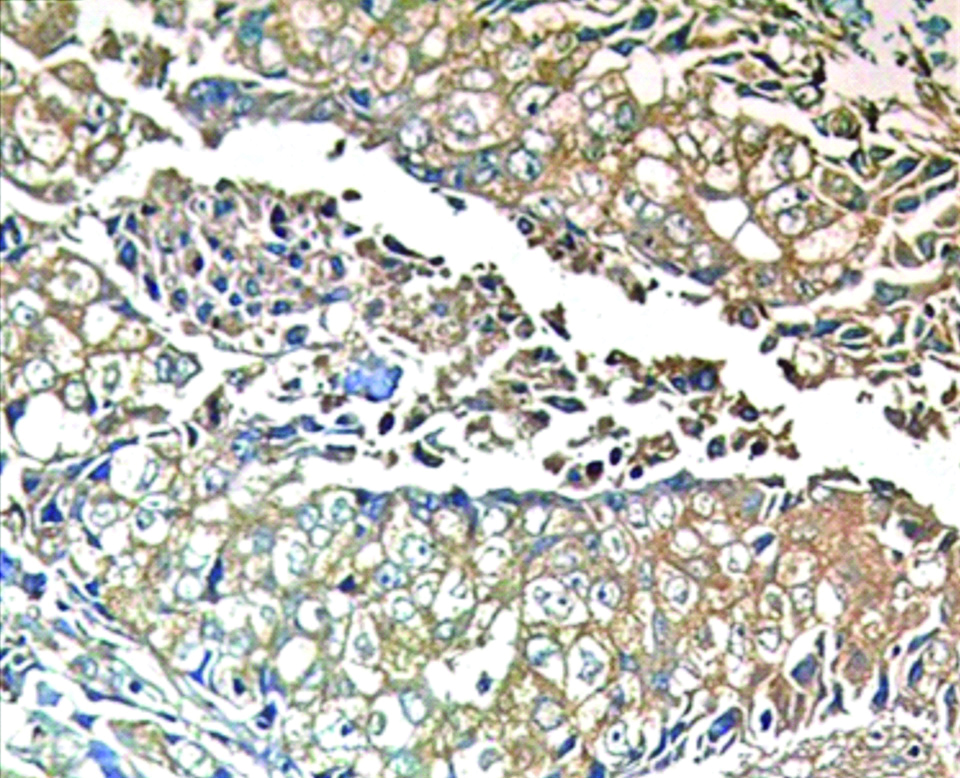
A highly statistically significant direct correlation was detected between TC and TILs PD-L1 expression (p-value≤0.001). The pathologic characteristics of the studied cases stratified by TC and TILs-PD-L1 expression were then summarised in [Table/Fig-7,8].
Immunohistochemical expression of both TC PD-L1 and TILs PD-L1 (×200).
The pathologic characteristics and PD-L1 protein expression status (Chi-square test).
| Parameter | | TC PD-L1 Positive (% within parameter) | TC PD-L1 Negative (% within parameter) | p-value | TIL PD-L1 Positive (% within parameter) | TIL PD-L1 Negative (% within parameter) | p-value |
|---|
| Histological type | IC-NST | 47 (61.8%) | 29 (38.2%) | 0.725 | 44 (57.9%) | 32 (42.1%) | 0.059 |
| ILC | 6 (60%) | 4 (40%) | 3 (30%) | 7 (70%) |
| Carcinoma with medullary features | 5 (71.4%) | 2 (28.6%) | 6 (85.7%) | 1 (14.3%) |
| Others | 3 (42.9%) | 4 (57.1%) | 2 (28.6%) | 5 (71.4%) |
| Histological grade | Low | 27 (47.4%) | 30 (52.6%) | 0.01* | 23 (40.4%) | 34 (59.6%) | 0.001* |
| High | 34 (79.1%) | 9 (20.9%) | 32 (74.4%) | 11 (25.6%) |
| Tumoural necrosis | Positive | 23 (79.3%) | 6 (20.7%) | 0.016* | 19 (65.5%) | 10 (34.5%) | 0.177 |
| Negative | 38 (53.5%) | 33 (46.5%) | 36 (50.7%) | 35 (49.3%) |
| T stage | T1+T2 | 49 (57.6%) | 36 (42.4%) | 0.102 | 44 (51.8%) | 41 (48.2%) | 0.122 |
| T3+T4 | 12 (80%) | 3 (20%) | 11 (73.3%) | 4 (26.7%) |
| N stage | 0 | 24 (53.3%) | 21 (46.7%) | 0.221 | 24 (53.3%) | 21 (46.7%) | 0.153 |
| 1 | 15 (57.7%) | 11 (42.3%) | 14 (53.8%) | 12 (46.2%) |
| 2 | 12 (70.6%) | 5 (29.4%) | 7 (41.2%) | 10 (58.8%) |
| 3 | 10 (83.3%) | 2 (16.7%) | 10 (83.3%) | 2 (16.7%) |
| Anatomic stage | Early (I and II) | 36 (54.5%) | 30 (45.5%) | 0.065 | 34 (51.5%) | 32 (48.5%) | 0.329 |
| Late (III and IV) | 25 (73.5%) | 9 (26.5%) | 21 (61.8%) | 13 (38.2%) |
| Prognostic stage | Early (I and II) | 24 (51.1%) | 23 (48.9%) | 0.008* | 20 (42.6%) | 27 (57.4%) | 0.004* |
| Late (III and IV) | 30 (78.9%) | 8 (21.1%) | 28 (73.7%) | 10 (26.3%) |
| ER | Positive | 40 (54.1%) | 34 (45.9%) | 0.016* | 34 (45.9%) | 40 (54.1%) | 0.002* |
| Negative | 21 (80.8%) | 5 (19.2%) | 21 (80.8%) | 5 (19.2%) |
| PR | Positive | 39 (55.7%) | 31 (44.3%) | 0.098 | 38 (54.3%) | 32 (45.7%) | 0.004* |
| Negative | 22 (73.3%) | 8 (26.7%) | 23 (76.7%) | 7 (23.3%) |
| Her2-neu | Positive | 12 (80%) | 3 (20%) | 0.102 | 13 (86.7%) | 2 (13.3%) | 0.007* |
| Negative | 49 (57.6%) | 36 (42.4%) | 42 (49.4%) | 43 (50.6%) |
| Ki-67 index | Low | 27 (54%) | 23 (46%) | 0.151 | 22 (44%) | 28 (56%) | 0.027* |
| High | 34 (68%) | 16 (32%) | 33 (66%) | 17 (34%) |
| BC subtypes | Luminal A | 13 (40.6%) | 19 (59.4%) | 0.006* | 10 (31.3%) | 22 (68.8%) | 0.002* |
| Luminal B | 27 (64.3%) | 15 (35.7%) | 24 (57.1%) | 18 (42.9%) |
| HER2 enriched | 10 (100%) | 0 (0%) | 8 (80%) | 2 (20%) |
| Triple negative | 11 (68.8%) | 5 (31.3%) | 13 (81.2) | 3 (18.8%) |
| LVI | Positive | 37 (75.5%) | 12 (24.5%) | 0.004* | 31 (63.3%) | 18 (36.7%) | 0.103 |
| Negative | 24 (47.1%) | 27 (52.9%) | 24 (47.1%) | 27 (52.9%) |
| Stromal TILs | Low | 44 (56.4%) | 34 (43.6%) | 0.076 | 36 (46.2%) | 42 (53.8%) | 0.001* |
| High | 17 (77.3%) | 5 (22.7%) | 19 (86.4%) | 3 (13.6%) |
*Statistically significant
Discussion
Programmed death-ligand 1 is an immune check point regulator up-regulated in many solid malignancies [14]. Antibodies targeting PD-L1 have shown clinical responses in multiple tumours [6]. Some of these antibodies have received FDA approval for clinical use [26]. Several studies investigated PD-L1 expression in BC, showing variable rates of expression within the tumour cells and the tumour infiltrating lymphocytes [27]. In BC, the PD-1 inhibitor Pembrolizumab has shown promising effects and a good safety profile [48]. PD-L1 inhibitors, atezolizumab and avelumab appear to be particularly active in TNBC [49].
In the present study, TC PD-L1 expression was reported in 61% of the cases. The rates of TC PD-L1 expression varied greatly in the literature ranging from 1.6% [20] to 94.9% [23]. However, many studies reported rates lower than the present; 34% [28], 8.3% [50] and 19.6% [51]. This variation can be explained by the large number of antibodies used, the use of full face sections versus Tissue Micro-Array (TMA), the different cut-off values used for positivity, whether cytoplasmic or membranous staining were interpreted and the composition of the studied population itself.
In our study, the relatively high rate of expression can be explained by the use of full face sections and a polyclonal antibody, as well as, the low cut off of positivity (1%). A study using full-face sections and a polyclonal antibody, similar to us, reported TC PD-L1 expression in 94.9% [23]. Another study using polyclonal antibody, but on TMA reported 56.5% positive TC PD-L1 expression [10]. The present choice of this polyclonal antibody stemmed from the absence of a uniformly accepted clone among the different organs.
Concerning the TILs PD-L1 expression, it was positive in 55% of the present cases. Similar to TC PD-L1 expression, a wide range of TILs PD-L1 expression rates have been reported in the literature, ranging from 6% [20] to 78% [8]. The rate of TILs PD-L1 expression in the present study (55%) was slightly lower than TC PD-L1 expression (61%). Although this agreed with some studies [31], many other studies reported the reverse [20,47,50].
Upon scoring our PD-L1 positive cases, 52.5% of the TC PD-L1 positive cases showed diffuse positivity (>50% of cells) and 32.7% of the TILs PD-L1 positive cases showed diffuse positivity (>50% of cells). Dill E et al., using the same scoring system reported diffuse TC PD-L1 expression in 20% of TC PD-L1 positive cases and diffuse TILs PD-L1 expression in 8.5% TILs PD-L1 positive cases [47]. Park I et al., reported positive PD-L1 expression of H-score 3+ in 51.6% of their cases [23] and Tomioka N et al., reported that 22.7% of the positive cases showed PD-L1 in >50% of the tumour cells [36].
Despite these variable figures, most of the studies agreed that a significant proportion of the positive cases showed only focal positivity, with considerable intra-tumoural heterogeneity of PD-L1 expression. Although the clinically relevant threshold of PD-L1 positivity for BC has not been yet established, this finding may be of significance if low levels of PD-L1 expression proved to be clinically actionable [47]. Caution should be exercised when interpreting PD-L1 stain on core biopsies where at least the negative cases may need repeated staining on later excision biopsies.
Regarding the histological subtypes, carcinoma with medullary features showed the highest rates of both TC and TILs PD-L1 expression, followed by IC-NST cases then ILC cases and finally other types (including mostly low grade types; papillary, mucoid, tubular and cribriform). This agreed with the results of Dill E et al., who reported the highest rates of PD-L1 expression in both compartments in cases with medullary features [47]. Guo L et al., and Hou Y et al., also reported higher rates of overall PD-L1 expression in IC-NST cases than ILC cases [41,52]. In addition, a study which analysed PD-L1 micro RNA (mRNA) expression in BC cell lines reported highest rates of PD-L1 expression in cases with medullary features [53].
In the present study, high grade cases showed statistically significant higher rates of TC and TILs PD-L1 expression than low grade cases. This is consistent with most of the studies in the literature [41,52,53]. Also, cases associated with necrosis showed higher TC PD-L1 and TILs PD-L1 expression than those devoid of necrosis. This was consistent with the detected high PD-L1 expression in high grade cases and in cases with medullary features.
Regarding the T stage, larger tumour size cases (T3 and T4) showed higher rates of TC and TILs PD-L1 expression than smaller tumour size cases. This was consistent with the results of Baptista MZ et al., and Li F et al., who reported higher TC PD-L1 expression in cases ≥ T2 [10,51]. In contrast, Park IH et al., and Kitano A et al., reported higher TC PD-L1 expression in smaller size cases. This may be explained by the fact that both those studies were concerned more by early stage BC cases with larger number of T1 and T2 cases than T3 and T4 cases [23,28].
In this study, cases positive for LN metastasis showed higher rates of TC and TILs PD-L1 expression than LN negative cases. This agreed with the results of a meta-analysis involving five studies [4] and another study [54]. Among the LN positive cases in the present study, the rate of TC PD-L1 expression increased with the increase in the N stage; N1 (57.7%), N2 (70.6%) and N3 (83.3%). This was consistent with the results of Qin T et al., [27].
On grouping our cases into anatomic and prognostic stages, the rate of TC and TILs PD-L1 expression was higher in advanced than early stage cases. This was consistent with the results of Qin T et al., and Kim A et al., who reported higher rate of TC PD-L1 expression in advanced anatomic stage cases [27,31]. However, Kim A et al., reported a contradictory higher rate of TILs PD-L1 expression in early stage cases [31].
It is to be noted that none of these studies staged their cases according to the recently published AJCC prognostic staging system. In the present study, only the correlations of PD-L1 expression with the prognostic stages, incorporating the more important grade and hormone status, were statistically significant.
Concerning the hormone receptors, higher TC and TILs PD-L1 expression was detected in hormone (ER and PR) negative then hormone positive cases. This was consistent with the results of Kim A et al., and Wimberly H et al., who assessed PD-L1 expression using quantitative immunofluorescence [31,55]. In contrast to the present results, Park H et al., reported statistically significant higher TC PD-L1 expression in hormone positive cases [23].
In the present study, the rate of TC and TILs PD-L1 expression was higher in the HER2 positive cases than in the HER2 negative cases. This agreed with the results of many studies [10,11]. This finding may be clinically important, where; some pre-clinical studies suggest that combining anti-HER2 therapy with some immune checkpoint regulators, such as anti-PD-1, anti-PD-L1 or anti-CTLA4 antibodies, may have a synergistic effect, increasing their antitumour efficacy [43].
Regarding the Ki-67 proliferation index, cases with high index showed higher rate of TC and TILs PD-L1 expression than low Ki67 cases. This result was consistent with the wide agreement on the association of PD-L1 expression with the high grade as well as with the results of some studies [11,56]. This correlation might be explained by the fact that high mutation rate of hyper-proliferative tumour cells in high Ki67 cases, may be responsible for higher immunogenicity due to the rapid emergence of neo-antigens [53].
Some other studies reported contradictory higher PD-L1 expression in low Ki-67 cases [23,27]. Those contradictory results again can be explained by the nature of Ki-67 itself as a marker of proliferation and its weak reproducible results.
On grouping the present cases into BC subtypes, the highest rate of TC PD-L1 expression was noticed in HER2 enriched subtype, while highest rate of TILs PD-L1 expression was noticed in triple negative subtype. Although, many studies reported highest PD-L1 expression in TN cases, the present results agreed with others [47]. Also, Cimino-Mathews A et al., reported highest both TC and TILs PD-L1 expression in HER2 enriched subtype [8]. Jang BS et al., also reported highest PD-L1 mRNA expression in HER2 enriched subtype. In general, almost all of the studies agreed that PD-L1 expression was higher in non-luminal than luminal BC [57].
In the present study, cases with high stromal TILs (≥30% of the stroma) showed higher rate of TC and TILs PD-L1 expression than cases with low stromal TILs. This agreed with the results of many others [4,8,42,55].
Finally, a highly statistically significant direct correlation was detected between TC and TILs PD-L1 expression in the present study. This was consistent with the results of many studies [8,31,47].
Limitation
One important limitation of this study is the lack of correlation with the patient’s prognosis and survival. However, the present study agreed with many others that PD-L1 expression is higher in tumours showing poor prognostic factors such as the high grade and lack of hormone receptors.
Conclusion
The present results are in favour of targeting the PD-1/PD-L1 pathway as an immunotherapy in BC, where, PD-L1 expression was detected in both BC tumour cells and TILs. The present authors reported that the expression of PD-L1 in BC is higher in a subset of tumours that are high grade, rich in TILs and lacking hormone receptors, suggesting that they may be important candidates for anti-PD-1/PD-L1 therapy.
*Statistically significant