Previous systematic reviews highlight the need to improve the oral health of caregivers and mothers to impact in their babies [1-3]. Preventive measures should be taught to the whole family to improve oral hygiene habits, based on instructions for oral health promotion [4-6]. Gingivitis is one of the most common clinical periodontal conditions among women during pregnancy and breastfeeding period [7-9]. It is well known that oral hygiene is related to gingival health [9], and the frequency of preventive measures during pregnancy as well as after the postpartum period, most of the time are neglected by women [7].
The aim of this study is to describe the oral health conditions and saliva profiles of mothers and their respective babies in the breastfeeding period, including the breast milk.
Materials and Methods
Sample Subjects
After approval of the Local Ethical Committee (CAAE: 0197.0.314.000-09), 47 pairs of mothers and babies were selected from two maternity hospitals and a outpatient public clinic ((Fernando Magalhães Hospital, Federal Hospital of Bonsucesso and the Department of Pediatric Dentistry and Orthodontics, School of Dentistry, Federal University of Rio de Janeiro). The sample was a convenience sample that was obtained during 19 months.
The inclusion criteria were: mothers breast-feeding with clinically healthy babies that were attending hospital for follow-up.
Exclusion criteria: smoking and/or the presence of systemic disease. Each woman signed a written consent authorising her own participation and also her babies participation-as legal guardians.
Data Collection
The study was cross-sectional, which analysed oral health status and profile of salivary metabolites of 47 women and their babies (0-28-months-old). The anamnesis of mothers and babies were performed in order to obtain information about age and socioeconomic conditions [7], as well as general health, delivery type, Body Mass Index (BMI) and breastfeeding. All participants provided 2 mL of unstimulated saliva by spitting into a sterilise plastic tube on ice. Mothers were required to refrain from eating and drinking for 2 hours and babies for 1 hour before saliva collection. For saliva collection of babies, a pipette was positioned at the floor of the mouth to collect 0.5 mL of unstimulated whole saliva. Thereafter, the breast milk was collected. We used the same methodology in the study as described by Fidalgo TK et al., [10]. During the collection period, the individuals were comfortably seated in a ventilated and lighted room. All salivary samples were centrifuged at 4°C and 10,000 g for 60 minutes (Cientec, CT-15000R, Brazil), and the supernatants were stored at −80°C until NMR (nuclear magnetic resonance spectroscopy) analysis as recommended by Silwood [11].
The intraoral examination was performed by a calibrated examiner, and periodontal exam was performed (Kappa=0.83), DMF-T suggested by the World Health Organisation [10] (Kappa=0.84). The mothers examination started with mucosa examination, followed by periodontal examination, which included the presence of plaque, gingival, calculus and suppuration for four surfaces and calculated percentages; Bleeding on Probing (BOP); Probing Pocket Depth (PPD) and Clinical Attachment Loss (CAL) recorded at six sites per teeth in all teeth (except at the third molars). Thereafter, data for DMF-T were collected [12,13]. It was considered with dental plaque subject with 25% of the surfaces with plaque, as suggested by the O’Leary index [14]. O’Leary index assess the oral hygiene status since it provide the percentages of biofilm. The sensitivity of this index is the oral hygiene level of each individual (above 25% the patient have a poor oral hygiene). Specificity of the index indicated by the amount of biofilm for each surface calculated according to the amount of teeth present in individual mouth [14]. Clinical diagnosis of periodontal status was established for all subjects based on the following criteria: periodontally healthy <10% of sites with BOP and no PPD or CAL >3 mm, although PPD or CAL=4 mm in up to 5% of the sites without BOP was allowed; gingivitis >10% of sites with BOP and no PPD or CAL >3 mm, although PPD or CAL=4 mm in up to 5% of the sites without BOP was allowed; chronic periodontitis >10% of teeth with PPD and/or CAL >5 mm and BOP. Generalised aggressive periodontitis was characterised by involvement of 30% of teeth with PPD and/or CAL >5 mm with BOP concurrently and including at least one incisor and first molar [15].
Regarding the babies, the oral examination consisted of bucal mucosa analyses to identify any mucosa changes, and, thereafter, the number of teeth and caries index were registered.
NMR Measurements
The salivary samples of mothers were prepared by mixing 0.450 mL of salivary supernatant, 0.050 mL of deuterium oxide (99.8% D2O, which provided a field-frequency lock) and 0.010 mL of sodium dodecyl sulphate 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) 20 mM for chemical shift reference of 1H spectra, δ=0.00 parts per million (ppm). On the other hand, the salivary samples of infants were prepared by mixing 0.170 mL of salivary supernatant, 0.0189 mL of D2O and 0.0038 mL of DSS 20 mM (maintaining the same proportion as the salivary samples of mothers for D2O and DSS preparation). The human milk samples were prepared by mixing 0.400 mL of salivary supernatant, 0.20 mL of D2O and 0.010 mL of DSS 10 mM for chemical shift reference of 1H spectra, δ=0.00 ppm. The field-frequency was set by detecting the D2O signal. For the NMR spectra acquisition, a Bruker 600 MHz Advance spectrometer (Bruker Biospin, Rheinstetten, Germany) equipped with a 5 mm high-resolution probe and operating at a frequency of 600 (1H) MHz was used [16]. All spectra were obtained at 25°C with water suppression by presaturation [16,17].
The Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence was used to suppress signals from proteins and other macromolecules through a T2 filter, (1024 scans for the salivary samples of mothers and 2048 scans for the salivary samples of infants). 1H-1H Total correlation spectroscopy (TOCSY) experiments were performed with acquisition parameters of 256 T1 increments, a spectral width of 12,019 Hz in each dimension and a mixing time of 70 ms. The major signals identify unambiguously assigned based on TOCSY, Silwood CJ et al., [11] and the Human Metabolome Database (http://www.hmdb.ca) [11,18].
Statistical Analysis
The data of mothers and babies, such as age, DMF-T index and the results from periodontal exams, were computed on SPSS software 16.0 (SPSS Inc., MN, USA). The frequencies, besides mean and Standard Deviation (SD), were described, and the Chi-square test was used for statistical analyses with a 5% significance level (p≤0.05). The chi-square test was done for the analyses of dental plaque against frequency of brushing/dental plaque against gingivitis. For analysis of the NMR data, each spectra was submitted to baseline adjustment by Topspin® software (Bruker Biospin, Rheinstetten, Germany), and the metabolite data were descriptively analysed.
Results
The mean±SD age of the mothers (n=47) and babies (n=48) was 27.6±6.34-years and 132.8±165.7-days, respectively. One mother had twins. Among the babies, 56% were male and 44% female. Thirty-nine (81.25%) babies were edentulous, while nine (18.75%) babies presented a total of 74 teeth, varying from 2 to 16 teeth each. None of them had dental caries. Three babies presented oral alterations, such as candidiasis (n=2) and Bohn nodules (n=1). More information such as weight, BMI of mothers, economic classification, parturition and breastfeeding are shown in [Table/Fig-1].
Demographic data which characterise the mother and babies and oral health condition.
| Variables | Mothers (n=47) | Babies (n=48) |
|---|
| Age (±SD)* |
| Mothers in years | 27.6 (±6.3) | 132.8 (±165.7) |
| Infants in days |
| Gender (%) |
| Male | --- | 27(56.2%) |
| Female | 21 (43.8%) |
| Income |
| <5 salaries | 46 (97.8%) | --- |
| 5-10 salaries | 0.0% |
| 10-15 salaries | 0.0% |
| >15 salaries | 1 (2.2%) |
| Economic classification 21 |
| B2 (score 23-28) | 1 (2.2%) | --- |
| C1 (score 18-22) | 20 (42.2%) |
| C2 (score 14-17) | 23 (48.9%) |
| D (score <14) | 3 (6.7%) |
| Weight-Kg (±SD)* | 66.6 (±18.2) | 3.3 (±6.2) |
| BMI (±SD)* | 26.1 (±5.8) | --- |
| Classification of BMI |
| Under weight | 3(5.10%) | --- |
| Normal weight | 19 (41.0%) |
| Overweight | 15 (33.4%) |
| Class I obesity | 6 (12.8%) |
| Class II obesity | 3 (5.1%) |
| Class III obesity | 1 (2.6%) |
| Breastfeeding (exclusive) | 30 (64.6%) |
| Parturition |
| Vaginal birth | 14 (29.2%) | --- |
| Cesarean section | 33 (70.8%) |
*Standard deviation; BMI: Body mass index
Considering the oral condition, the presence of dental plaque was observed in 62% of mothers and 72.4% presented with gingivitis [Table/Fig-2]. No difference was found between the correlation of dental plaque and frequency of dental brushing as well as the gingivitis. The DMF-T index was 8.20 (±6.39) and the decayed component was 1.71 (±1.94), indicating low levels of actual caries activity with a significant caries history.
Oral findings of mothers and their babies.
| Variables | Mothers group (n=47) |
|---|
| Frequency of tooth brushing (times a day) | 2.7±0.8 |
| Plaque presence* | 29 (62.0%) |
| Presence of calculus | 3 (6.1%) |
| Pocket depth (mm) | 2.0 |
| Bleeding on probing (% sites) | 22 |
| Attachment level (mm) | 2.1 |
| Suppuration (% sites) | 0.0 |
| Gingivitis (% women)** | 34 (72.4%) |
| Periodontitis (% women)*** | 0% |
| DMFT (±SD)**** | 8.2 (±6.39) |
| Decayed teeth | 1.71 |
| Missing teeth | 16.4 |
| Filled teeth | 9.9 |
| Variables | Babies group (n=48) |
| Number of teeth (±SD)**** | 3.2±6.3 |
| Babies without teeth | 39 (81.2%) |
| Babies with teeth | 9 (18.7%) |
| Dmft | 0.0 |
| Oral healthy (%) | 45 (93.8%) |
| Alterations | |
| Candidiasis (%) | 2 (4.2%) |
| Bohn nodule (%) | 1 (2.1%) |
*62.0% of the mothers had over 25% plaque presence (O’Leary et al., 1972); **72.4% of the mothers had gingivitis (da Silva-Boghossian et al., 2011); ***(da Silva-Boghossian et al., 2011); ****Standard deviation
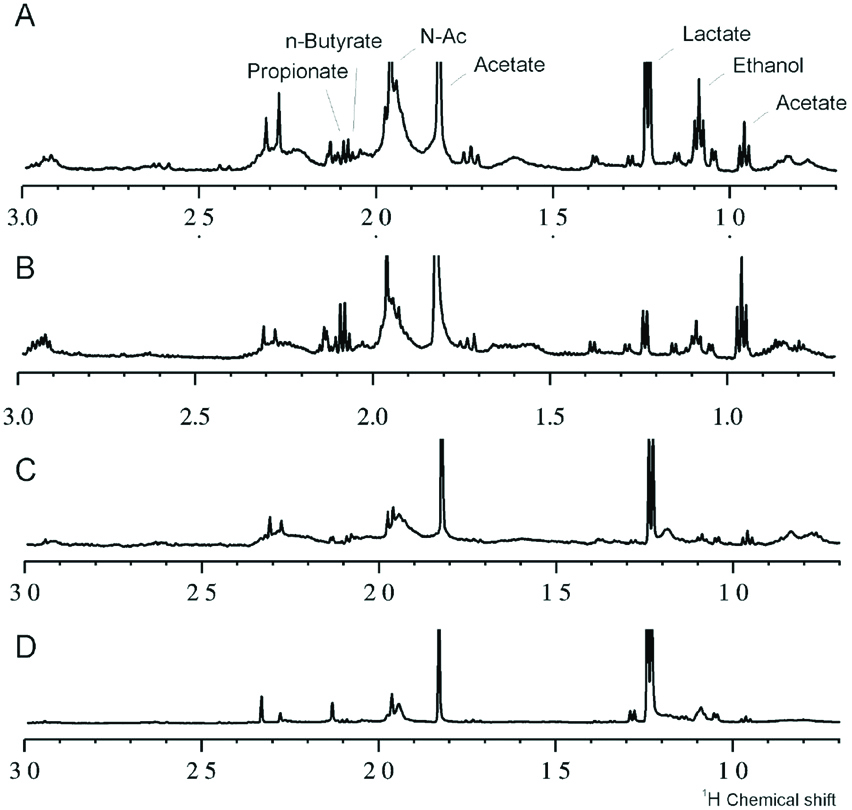
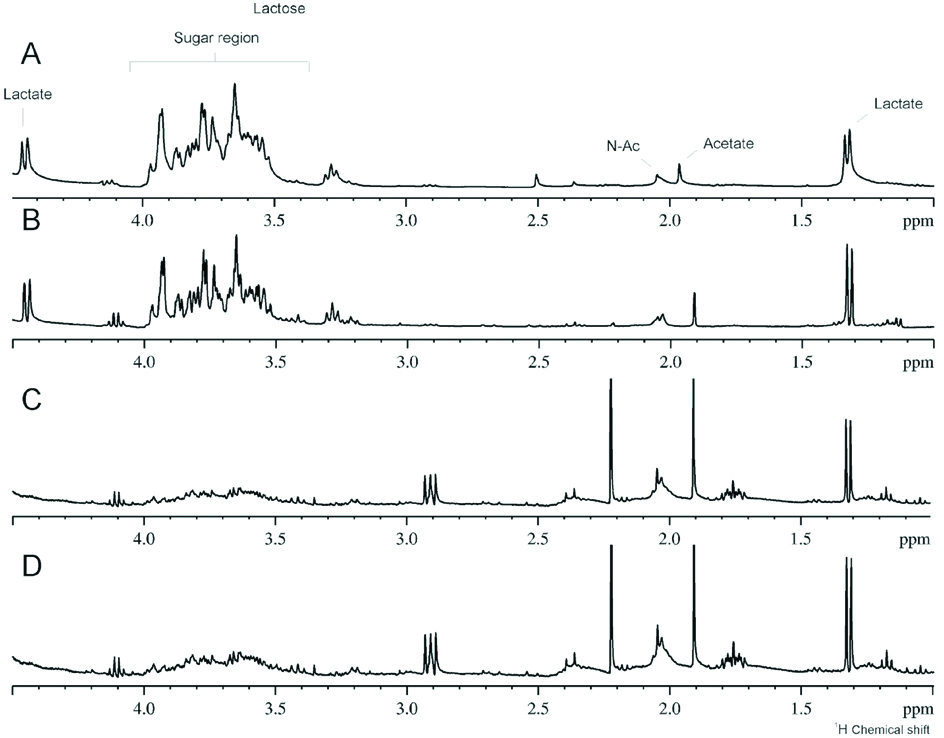
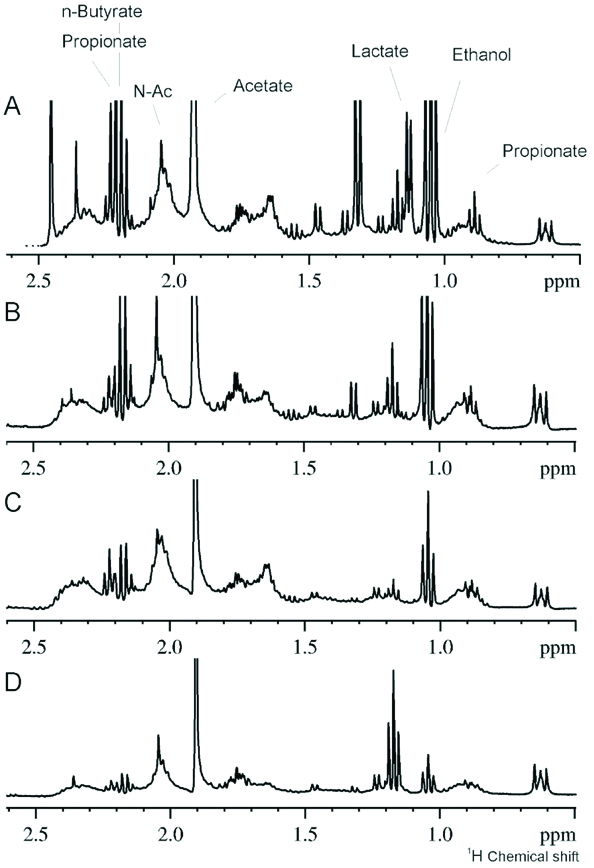
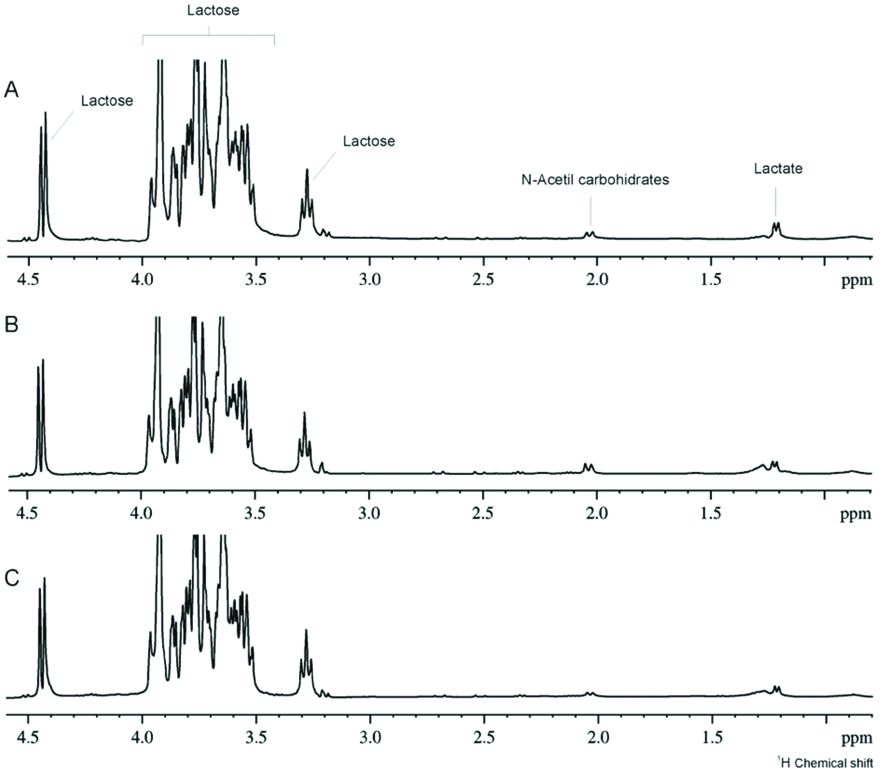
The [Table/Fig-3] shows that babies with more than six months of age, with teeth, had increasing levels of lactate, ethanol, acetate, propionate, N-butyrate and N-acetyl sugars and reduced levels of sugar. The [Table/Fig-4] demonstrates the spectra of children without teeth with exclusive and mixed breast milk, showing the presence of milk in saliva. The [Table/Fig-5] shows 1H-NMR analysis of salivary samples from orally healthy and unhealthy mothers (caries or periodontal disease), showing alterations in metabolites, such as propionate, ethanol, lactate, acetate, butyrate and N-acetyl sugar, and the sugar region. Finally, the [Table/Fig-6] illustrates the 1H-NMR breastfeeding milk from three mothers, demonstrating the high intensity of lactose in the sugar region.
1H NMR saliva spectra differences among children with (A and B) and without teeth.
1H-NMR saliva spectra of children without teeth with exclusive (A and B) and mixed (C and D) breast milk.
1H NMR saliva spectra of mothers with (A and B) and without (C and D) oral disease.
Illustrates the 1H-NMR breastfeeding milk from three mothers, demonstrating the high intensity of lactose in the sugar region.
Discussion
Oral hygiene is an important step in the maintenance of oral health, especially during the pregnancy and breastfeeding period [19,20]. Mothers of young children are supposed to understand that their oral health influence the health of their babies [19,21]. In our study, the mothers had high amounts of dental plaque and gingivitis, which was not consistent with the frequency of oral hygiene habits mentioned by them. The level of gingivitis in this group is in line with previous investigations and correlates with the amount of dental plaque [22]. Gingivitis is most frequent periodontal disease observed among women during the breastfeeding period [23-25], this can be related to the hormonal changes that occur during gestational as well as puerperal period [24,26]. According to the applied criteria in this study [7], we could not classify any patient with periodontitis.
The present study evaluated the mother’s oral condition by dental caries score and oral health related to their children’s oral status. Although the subjects were young, the missing teeth component was high, indicating a decayed oral history. It was also correlated with previously study that indicated the number of missing teeth in the mothers reflected the number of decayed teeth of their children [26]. One of the limitations of our study is the small sample size but despite of that we found similar results in other study done with larger population [27]. Dental care should start during the early childhood and the mothers are key participants in the process, while the school and community could give important support [28]. The main finding of the present study is that mother’s oral health is not reflected in the children’s oral health due to the young age, IgA from the milk among other factors, although they claim lack of daily oral hygiene [20,29].
The oral conditions of the babies were mostly healthy; one child presented Bohn nodules, and two children presented candidiasis, a well-documented condition in children [30]. It is important to note that both children with candidiasis are twins and the mother was not aware of the necessity of cleaning breast and mouth after feeding each baby or even if this condition needed treatment. The baby twins were treated and recovered oral health. This fact motivates the mother to understand the impact of baby mouth cleaning particularly during and after the breastfeeding period. The best way to clean the babies’ mouth is still under debate, despite the importance of preventing even opportunistic flora. Sales AB et al., compared cleaning with gaze and hydrogen peroxide (0.75%) or distilled water and observed no difference in the reduction of the number of Streptococcus spp and Candida [31].
Our study showed similarities among mother/babies saliva by NMR analysis. However, it seems that baby saliva has higher amount of sugar, such as lactose because of breast milk. It was also found that a modification of saliva profile occurred in 1 month and 6 months old babies, which revealed changes in the levels of lactate, ethanol, acetate, propionate, N-butyrate and N-acetyl sugars and reduced levels of sugar. It is known that the saliva flow rate of young children is 18 times lower than older children or adults [32]. The saliva of the younger babies, exclusively breastfeeding, presented increased levels of lactose, and low levels of acid production and older babies saliva, non-exclusively breastfeeding, we observed a decrease in sugar content.
The reduced saliva flow rate in babies is a factor for bacteria retention on different oral surfaces, which favors growth of bacteria. It is understood that baby saliva could play a role in the colonisation of bacteria in the mouths of young children. Further investigation should be done to clarify the composition of baby saliva. The children with primary dentition presented with a higher concentration of organic acids from the metabolism of microorganisms. These children presented erupted teeth and higher areas of enamel for microorganism colonisation, increasing the acid production [10].
In addition, a preventive programme that would improve the oral health and quality of life should include an educational program involving the family, community and health personnel. There is an impact of maternal self-efficacy and oral health beliefs on early childhood caries and other factor as for example cultural background may influence this aspect, as was pointed out observed by Wilson AR el al., 2017 [20]. Consequently, the programme should incorporate caregiver’s social determinants including knowledge, health beliefs and attitudes, stress, self-efficacy, social network strength, and acculturation status [29]. Since, higher sugar consumption is well correlated with higher caries risk, as was indicated in the recommendation for oral health in infancy (AAPD 2014), the role of the family should be the change in lifestyle and exchange the high sugar level diet to healthier one, and therefore, to promote adequate child development (WHO 2017) [30,33].
Limitation
Small sample size and similar socio-economic status that may have affected the results.
Conclusion
The mothers that participated in this study had poor oral health, which didn’t had an impact on the oral health of their babies. NMR analyses of saliva samples showed differences between mothers and babies, and the intensity of metabolites, showed that lactose is dominant in the saliva of younger babies when compared to mothers and older babies. Non-exclusive breastfeeding babies’ saliva had less sugar content. It seems that babies saliva composition is related to the diet.
Future Recommendation
The mother should be aware of the impact of baby mouth cleaning particularly within and after the breastfeeding. The best way to clean the babies’ mouth is still under debate. It is necessary to design a preventive approach for mother/babies during breastfeeding period to improve early childhood quality of life.
*Standard deviation; BMI: Body mass index
*62.0% of the mothers had over 25% plaque presence (O’Leary et al., 1972); **72.4% of the mothers had gingivitis (da Silva-Boghossian et al., 2011); ***(da Silva-Boghossian et al., 2011); ****Standard deviation