Introduction
Proper nutrition is considered to have constructive functional and structural effects on brain. As a fact, brain has its own life cycle in physiological, biochemical and anatomical evolution [1]. The daily required protein in childhood is 1.2 gm/kg body weights and 10-16% of daily body calorie is obtained from the proteins [2]. Protein deficiency may affect the chemical structure of brain neurons and even behaviour [3]. There are a lot of unanswered questions regarding how improper diet can develop neurological and psychological disorders [4].
The relationship between a perfect diet and appropriate evolution of the brain, specifically in children, has always been discussed [5]. The natural evolution of different brain regions starts from the neonatal period until after birth and includes numerous brain cells, migration and organisation of neurons [6]. Development of neuritis includes the formation of dendrites and axons fibres depending on the nature of the stimulus received by the brain and the quality of an appropriate diet [7]. The structure of myelin, glial cells, and neurons are rich in protein; therefore, an appropriate diet, specifically full of proteins, is required for the evolution of different brain regions [8].
The biosynthesis of brain proteins depends on the efficient and consistent intake of amino acids. Furthermore, proteins are necessary for supplying the required energy for metabolism and synthesis of neurotransmitters in the brain [9]. A hippocampus with its internal structure plays a vital role in long-term memory formation, and its function is associated with a stimulation reward system [10]. The hippocampus as a part of the limbic system is essential in the formation of different types of learning and memory [11]. CA1 area, with a vigorous role in changing short-term memory to long-term, belongs to the hippocampus [12]. The published results of Kinzig KP et al., indicated that due to low protein diet in rats, the malformation in the hypothalamus nucleus occurs [13]. With regard to the importance of protein in brain evolution, very little knowledge is currently known about the impacts of HPD on learning and changes in CA1 region neurons. Thus, the current study was designed to investigate the impacts of HPD on neurons changes of the CA1 region and the level of learning in rats.
Materials and Methods
Experimental animals: The present experimental study was carried out in the Anatomy Department of the Kermanshah University of Medical Faculty from May 2018 to December 2018. Thirty male (Wistar rats despite the sensitivity of female mice, the presence of female sex hormones and their increase and decrease during the study can affect the results, therefore, male rats were used in this study) 220-240 gm and eight-week-old (were prepared from the Pasteur Institute and transferred to the animal house in medical school. The rats were kept under standard conditions (12 hour/12 hour light/dark cycle and 22±2°C with a relative humidity of 50±5%), in special cages and on a straw bed. Water and standard food were freely available to the animals including plate and treated municipal water. The research confirmed to the Ethical and Human Principles of Research and was approved by the Ethics Committee (ethics certificate No. 97497).
Grouping and diet: A total of 20 male animals were separated by random samplling (Initially, the number of animals in each group was 15 (a total 30 rats) and during the study 5 rats died in each group. In the control group, a normal diet including 14% protein was used. In HPD group high protein including 35% of protein was used. The required protein-rich food for both groups was provided from Pars Animal Food Company in Tehran, Iran. The feeding was followed for 10 months. The required food was 5-6 gm daily per 100 gm of the weight of rat. The diet contained 500 gm total milk protein per kg of food. Total milk protein is a mixture of casein (85%) and other milk proteins (albumins and globulins). These other milk proteins constitute a direct source of limiting sulfur amino acids. In HPD diet, protein was added at the expense of an energy equivalent amount of sucrose and starch. Base energy in both diets was similar. In the first group with normal diet, base energy was 2531 kcal, and in the second group with HPD, base energy was 2534 kcal. Energy-to-protein ratio in the first group was 145 and 317 in the second group [14,15].
Weight of animals: The body weight was measured using a microbalance sensitive up to 0.1 mg (Precisa 125A; Switzerland) [16].
Transcardiac perfusion: Rats were injected with a mixture of Xylazine (10 mg/kg) and Ketamine (70 mg/kg) to induce general anaesthesia. The thoracic cage was cut anteriorly and the left ventricular apex was observed. Aorta was detected and fixed with a 1 mm glassy cannula inside the lumen. The left ventricle was dissected to reach the cannula in the ascending aorta, and then the descending aorta was clamped just superior to the diaphragm. Normal saline was injected into the cannula. The pericardium and the right ventricle were cut. The left ventricle pathway was dissected and the ascending aorta was connected to a plastic tube by the glassy cannula and descending aorta was clamped right above the diaphragm. The cannula linked to the saline solution was implanted into the aorta through an incision in the left ventricle. The pericardium and the right ventricle were cut. The left ventricle pathway was dissected and the ascending aorta was connected to a plastic tube by the glassy cannula and descending aorta was clamped right above the diaphragm. The cannula linked to the saline solution was implanted into the aorta through an incision in the left ventricle. The descending aorta was clamped and after brain washing, the solution was discharged through the incision made in the right atrium. Formalin 5% and buffer phosphate 7% were infiltrated into the brain by a cannula and the brain was fixed in 15 min. After perfusion, the brains were dissected and stored in a similar perfusion solution for 3 days [11].
Shuttle box method: The Passive-Avoidance Learning ability of rats was assessed by the means of a shuttle-box device. The device contained two isolated compartments (20×30×20 cm3) dividing through a guillotine entrance from which. When it is open, the rats could cross through that. The floor and the walls of one of the compartments were white and for another one was black. The base of both rooms had parallel metal bars through which electrical stimulation with favourite time and voltage could be transmitted to animals’ feet via an attached stimulator. Passive-avoidance learning was assessed in three phases based on usual technique [10].
Cresyl violet method: Cresyl Violet staining was used to determine the number of living cells in the hippocampus CA1 region. For this purpose, six mice in each group was decapitated and five histological sections were prepared from every rat. Subsequently, 5 μm cuts were obtained using microtome, tissue processing performed, and the left hemispheres were stained using Cresyl Violet staining technique. In brief, the slips were stable again (10 minutes) in 4% paraformaldehyde solution. Slide was immersed in 70% (5 minutes) and 100% (15 minutes) ethanol and in xylene for 20 minutes. They were then immersed back through the ethanol descent concentrations. They were stained for 5 minutes in filtered Cresyl Violet solution, and then washed in distilled water. The slides were then dehydrated again in ethanol. They were located in xylene for another 10 minutes and then cover slipped. After photo preparation, the number of cells was counted in one square millimetre. The round cells stained with Cresyl Violet with no peak nose were considered as living cells [11].
Dendritic spines: The dendritic thorns were counted via an optical microscopic examination and software of Motic and Image tool IT (version 3). In the Golgi-stained slides, the neurons which entirely stained as the position of cell bodies in the central part of the tissue slices take distance from the surrounding stained neurons and they constituted the inclusion criteria. The dendritic tree of pyramidal neurons was demonstrated through a Camera Lucida at 400X magnification and the dendritic exclusion order from the cell body was used for counting the dendritic pieces [11].
Golgi method: The Golgi method was used to observe the neuron dendrites in the hippocampus CA1 region. This method was applied using potassium dichromate followed by silver nitrate. After brain fixation, the tissue blocks were suspended inside 3% potassium dichromate solution in a dark environment for 48 hours. After washing the blocks in 0.75% silver nitrogen, they were suspended in the solution for three days. The washing of the tissues was carried out in 1% silver nitrate solution. Now the tissue is ready for processing, dehydration, cleansing and embedding. The histological slides with a thickness of 5 μm were prepared for staining and morphological examination [12].
Statistical Analysis
Kolmogorov-Smirnov test was first conducted to confirm the data compliance for normal distribution. Then t-test was used for statistical analysis and determination of differences between the groups. SPSS version 16 was used for data analysis. The obtained results were expressed as mean±standard error and p-value <0.05 was considered statistically significant.
Results
Body weight: HPD showed that the mean of the animal’s weight increased compared to the control group (p-value <0.05) [Table/Fig-1].
Different weight of animals and active-avoidance learning ability between treatment groups.
| Factors | Control | HPD |
|---|
| Mean of rats weight (g) | 220±20 | 270±10* |
| Learning ability (s) | 425.5±15.70 | 275±4.19* |
| Mean number of neuron (%) | 18.33±0.60 | 28.19±0.30* |
| Mean number of dendritic spines (%) | 14.66±0.24 | 27.13±0.16* |
Data were presented as mean±standard deviation; *p<0.01 compared to the control group; HPD: High protein diet
Active-avoidance learning ability: The outcomes of the shuttle-box apparatus showed active-avoidance learning. The ability of HPD group was significantly increased compared to the control group (p-value <0.05) [Table/Fig-1].
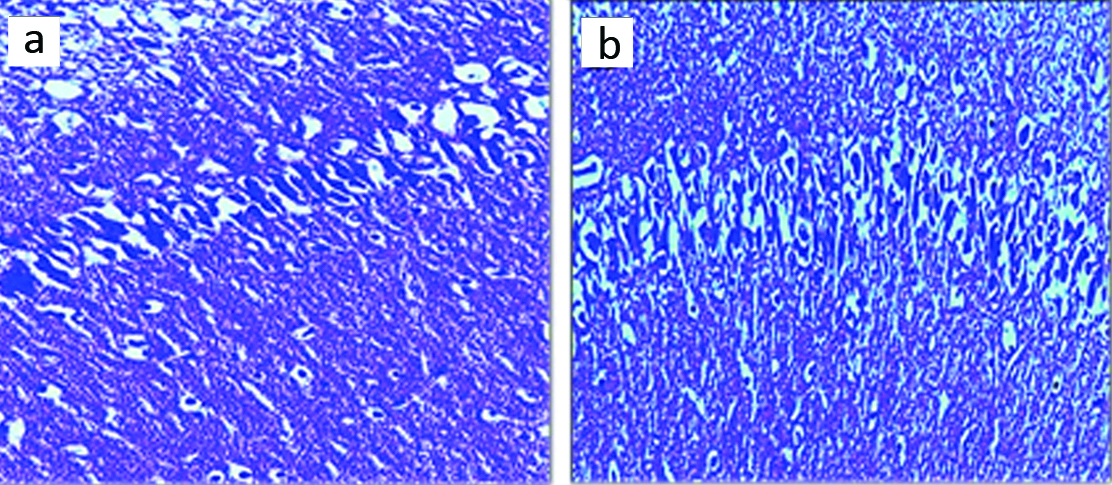
Neuron numbers: The results of neuron counting in the hippocampal region CA1 showed a significant increase in HPD group compared to the control group (p-value <0.05) [Table/Fig-2a,b].
Microscopic images of the mean number of neuron in CA1 region of male rats in different groups (5 μM thick sections, Cresyl Violet staining, at X100 magnification). Micrograph of the CA1 section in the control group (a), the normal number of neurons; Micrograph of the CA1 section in HPD diet group (b), rise in the number of neurons cells can be seen.
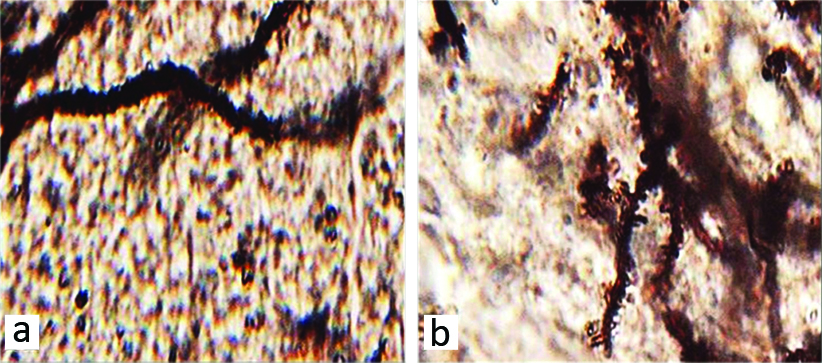
Dendritic spines: The mean number of neuronal dendritic spines showed a significant increase between the control and HPD group (p-value <0.05) [Table/Fig-1,3a,b].
Microscopic images of the mean neuronal dendritic spines in hippocampal region CA1 in different groups (Five-micron thick sections, Golgi staining, magnification X100). Micrographs of the CA1 section in the control group (a), normal structure. Micrograph of the hippocampal region CA1 section in HPD group (b), increased number of dendritic spines.
Discussion
The present study investigated the impacts of HPD on neurons changes available in hippocampus region and amount of learning in rats. The results of this study indicated that HPD increased the total weight of the rats significantly. Since some sources of energy and calorie supply in the body are provided by proteins, but a rise in the amount of received calories is considered to be one of the reasons for weight gain in rats [17]. The intracellular amino acids switch to structural and functional proteins while other additional amino acids undergo metabolism to release energy or store as adipose tissue [18]. It is believed that the reduced amount of proteins has critical importance in creation of malformation of different parts of the body and also in the regeneration of cell and tissue [19]. According to a study, when the body receives a high amount of protein, a specific percentage of proteins do not break down to amino acids leading to the weight gain of rats [20]. The published results of Shiell AW et al., are consistent with the results of the present survey indicating that HPD in pregnancy causes severe weight gain of rat newborn [21]. This growth in body fat due to a HPD can be partially explained by a 19% rise in energy intake [22]. The finding of this research together with previous studies of Jean C et al., in animals show that an increase in protein intake is more satiating than carbohydrate and fat on a weight-to-weight basis [23].
The results of the current study showed that the number of neurons and dendritic spines increased significantly in HPD group in comparison with the control group. The results may indicate a reduction of the apoptosis process and neurodegeneration by administration of HPD [24]. The results of Mattson MP et al., were consistent with findings of the present study show that the HPD could protect the cells in the brain by increased protein accumulation in the membrane and increase the cell size [25].
It seems that long-term HPD prevention will induce oxidative stress status. The production of free radicals such as superoxide and hydroxyl radicals will occur, which can cause cell damage [26]. Generated free radicals following oxidative stress may have the potential to damage the cellular compositions including proteins, lipids, and DNA [27]. The results of this experimental survey are in line with the Andrade JP et al., results which confirm the decrement effect of LD in the volume and number of neurons exist in the subclavian area [28]. It has been proved that the main factor in the process of synaptic transmission is related to the dendritic thorns. For this reason, the cause of many brain diseases returns to a change in morphology and dendritic thorn density [12]. HPD can grow the length and the number of dendritic spines in Nucleus Accumbens by affecting the neurotrophic factors in the Striatum [29]. A study by Dabydeen L et al., showed that HPD could increase the length and density of synaptic thorns; that is consistent with the results of the present study [30]. The results may indicate the ability of control in apoptosis and neurodegeneration processes by administering of HPD compared to the normal diet [31]. HPD in long term usage prevents the reactive oxygen species activity and lipid peroxidation and has the potential to destroy the oxidative stress compared to normal protein diet [32]. It seems that HPD decreases the expression of Cyclin B1 and Bax protein (involved in cell cycle and apoptosis) [33]. Although the precise biological mechanism behind the HPD-induced neurotic improvement is unclear, the psychopharmacological and sedative activity of HPD can help to describe the improvement of CA1 dendritic compared to the normal diet which is in line with the results of Sarfert KS et al., [34].
The reduction of protein can destroy the dendritic thorns in post-synaptic cell of hippocampus region by β2-nAChRs deactivation [35]. Moreover, the protein deficiency can reduce the number of thorns by two processes, deactivation of α4β2-nAChRs in the pre-synaptic membrane and disruption in release of glutamate neurotransmitters [36]. It appears that the protein deficiency which induces the cellular destruction in CA1 region is related to amplified expression apoptosis and cell cycle markers such as Cyclin B1 which can interfere with neuronal death [37]. HPD in long-term administration inhibits the lipid peroxidation of quinolinic acid and control the production of cyanide-induced superoxide [33]. HPD can increase the effects of antioxidant enzymes such as catalase and superoxide dismutase and reduce ROS production compared to the normal protein diet [38]. HPD can prevent the stimulation of nitrite oxide receptors in the brain and decrease the release of glutamate and NMDA activation. The activation of NMDA may increase the formation of nitrite oxide in the brain cortex [39]. Administration of HPD could inhibit nitrite oxide production [40].
The results of the current study showed that the HPD have useful effects on the learning ability as indicated by the active-avoidance test. Growth in the learning ability has been reported by clinical studies in adults with HPD [41]. Beneficial results of this study achieved in long-term HPD consumption including a rise in amount of learning in the rats created by a rise in the number of neurons and their dendrites which consequently increased the density of synapses in CA1 regions of hippocampus [42]. Proteins are one of the main factors in neurotransmitters production. So, its deficiency can cause serious disorder in the function of synapses in hippocampus [43]. Reyes-Castro LA et al., reported that the increase in the amount of protein in daily diet improved the spatial memory which is in line with the results of the current study [44]. According to the current data, administration of HPD significantly improved learning deficits compared to the normal diet. Frankincense is known as an effective anti-inflammatory agent. Calcium is the main stimulus for freeing neurotransmitters, so it plays a vital role in the synaptic facilitation [45]. It has been shown that the HPD cause displacement of calcium. This plays a role in the synaptic enhancement in hippocampus [11]. Also, can be assumed that HPD advances spatial memory through affecting the metabolism of arachidonic acid compared to the LD diets [46].
Limitation
The present study has certain limitations; authors did not study in detail about the mechanism of HPD on the CA1 region There were death of some animals due to long timeline (10 months) of this study. Hence prospective studies should be taken for detailed association of the molecular interaction between HPD and CA1 region.
Conclusion
HPD has useful effects on the CA1 region. HPD may increase the learning ability and has progressive effects on animals. As a result, HPD leads to a growth in the learning ability through CA1 tissue rise and increasing the number of neurons, and dendritic spines.
Data were presented as mean±standard deviation; *p<0.01 compared to the control group; HPD: High protein diet
[1]. Andrew MJ, Parr JR, Montague-Johnson C, Laler K, Qi C, Baker B, Nutritional intervention and neurodevelopmental outcome in infants with suspected cerebral palsy: the Dolphin infant double-blind randomized controlled trialDev Med Child Neurol 2018 60(9):906-13.10.1111/dmcn.1358629023666 [Google Scholar] [CrossRef] [PubMed]
[2]. Pasini E, Corsetti G, Aquilani R, Romano C, Picca A, Calvani R, Protein-amino acid metabolism disarrangements: the hidden enemy of chronic age-related conditionsNutrients 2018 10(4):391-97.10.3390/nu1004039129565819 [Google Scholar] [CrossRef] [PubMed]
[3]. Singh K, Lilleväli K, Gilbert SF, Bregin A, Narvik J, Jayaram M, The combined impact of IgLON family proteins Lsamp and Neurotrimin on developing neurons and behavioral profiles in mouseBrain Res Bull 2018 140:05-18.10.1016/j.brainresbull.2018.03.01329605488 [Google Scholar] [CrossRef] [PubMed]
[4]. Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Dietary intake of antioxidants and risk of Alzheimer diseaseJAMA 2002 287(24):3223-29.10.1001/jama.287.24.322312076218 [Google Scholar] [CrossRef] [PubMed]
[5]. Hooper C, Barreto PD, Pahor M, Weiner M, Vellas B, The relationship of omega 3 polyunsaturated fatty acids in red blood cell membranes with cognitive function and brain structure: A review focussed on Alzheimer’s diseaseJ Prev Alzheimers Dis 2018 5(1):78-84. [Google Scholar]
[6]. Adhya D, Annuario E, Lancaster MA, Price J, Baron-Cohen S, Srivastava DP, Understanding the role of steroids in typical and atypical brain development: advantages of using a “brain in a dish” approachJ Neuroendocrinol 2018 30(2):e1254710.1111/jne.1254729024164 [Google Scholar] [CrossRef] [PubMed]
[7]. Jalili C, Salahshoor MR, Khademi F, Jalili P, Roshankhah SH, Morphometrical analysis of the effect of nicotine administration on brain’s prefrontal region in male ratInt JMorphol 2014 32(3):761-66.10.4067/S0717-95022014000300003 [Google Scholar] [CrossRef]
[8]. Southam KA, Stennard F, Pavez C, Small DH, Knockout of Amyloid β Protein Precursor (APP) expression alters synaptogenesis, neurite branching and axonal morphology of hippocampal neuronsNeurochem Res 2018 23(6):01-10.10.1007/s11064-018-2512-029572646 [Google Scholar] [CrossRef] [PubMed]
[9]. Nesterkina M, Bernier UR, Tabanca N, Kravchenko I, Repellent activity of monoterpenoid esters with neurotransmitter amino acids against yellow fever mosquito, AedesaegyptiOpen J Med Chem 2018 16(1):95-98.10.1515/chem-2018-0015 [Google Scholar] [CrossRef]
[10]. Jalili C, Pourmotabbed A, Moradi S, Salahshoor MR, Motaghi M, The therapeutic effect of the aqueous extract of Boswelliaserrata on the learning deficit in kindled RatsInt J Prev Med 2014 5(5):563-68. [Google Scholar]
[11]. Moradi S, Pourmotabbed A, Salahshoor MR, Jalili C, Motaghi M, Kakebaraei S, The Morphometric Effects of Aqueous Extract of Boswellia Serrata on Hippocampal Region CA1 in kindled RatInt J Morphol 2014 32:1271-76. [Google Scholar]
[12]. Jalili C, Salahshoor MR, Pourmotabbed A, Moradi S, Motaghi M, Darehdori S, The effects of aqueous extract of boswellia serrata on hippocampal region CA1 and learning deficit in kindled RatsRes Pharm Sci 2014 9(5):351-58. [Google Scholar]
[13]. Kinzig KP, Hargrave SL, Hyun J, Moran TH, Energy balance and hypothalamic effects of a high-protein/low-carbohydratedietPhysiolBehav 2007 92(3):454-60.10.1016/j.physbeh.2007.04.01917512959 [Google Scholar] [CrossRef] [PubMed]
[14]. Langley SC, Jackson AA, Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein dietsClinsci 1994 86(2):217-22.10.1042/cs08602178143432 [Google Scholar] [CrossRef] [PubMed]
[15]. da Rosa Lima T, Ávila ET, Fraga GA, de Souza Sena M, de Souza Dias AB, de Almeida PC, Effect of administration of high-protein diet in rats submitted to resistance trainingEur J Nutr 2018 57(3):1083-96.10.1007/s00394-017-1391-528236109 [Google Scholar] [CrossRef] [PubMed]
[16]. Salahshoor MR, Roshankhah S, Hosseni P, Jalili C, Genistein Improves liver damage in male mice exposed to morphineChin Med J 2018 131:1598-604.10.4103/0366-6999.23511729941714 [Google Scholar] [CrossRef] [PubMed]
[17]. Annegers JF, The protein-calorie ratio of West African diets and their relationship to protein calorie malnutritionEcol Food Nutr 1973 2(3):225-35.10.1080/03670244.1973.9990340 [Google Scholar] [CrossRef]
[18]. Albrechtsen NJ, Færch K, Jensen TM, Witte DR, Pedersen J, Mahendran Y, Evidence of a liver–alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acidsDiabetologia 2018 61(3):671-80.10.1007/s00125-017-4535-529305624 [Google Scholar] [CrossRef] [PubMed]
[19]. Zhang X, Farah N, Rolston L, Ericsson DJ, Catanzariti AM, Bernoux M, Crystal structure of the Melampsoralini effector AvrP reveals insights into a possible nuclear function and recognition by the flax disease resistance protein PMol Plant Pathol 2018 19(5):1196-209.10.1111/mpp.1259728817232 [Google Scholar] [CrossRef] [PubMed]
[20]. Adibi SA, Metabolism of branched-chain amino acids in altered nutritionMetabolism 1976 25(11):1287-302.10.1016/S0026-0495(76)80012-1 [Google Scholar] [CrossRef]
[21]. Shiell AW, Campbell-Brown M, Haselden S, Robinson S, Godfrey KM, Barker DJ, High-meat, low-carbohydrate diet in pregnancy: relation to adult blood pressure in the offspringHypertension 2001 38(6):1282-88.10.1161/hy1101.09533211751704 [Google Scholar] [CrossRef] [PubMed]
[22]. Belobrajdic DP, McIntosh GH, Owens JA, A high-whey-protein diet increase body weight gain and alters insulin sensitivity relative to red meat in wistar ratsJ Nutr 2004 134(6):1454-58.10.1093/jn/134.6.145415173411 [Google Scholar] [CrossRef] [PubMed]
[23]. Jean C, Rome S, Mathé V, Huneau JF, Aattouri N, Fromentin G, Metabolic evidence for adaptation to a high protein diet in ratsJ Nutr 2001 131(1):91-98.10.1093/jn/131.1.9111208943 [Google Scholar] [CrossRef] [PubMed]
[24]. Mattson MP, Apoptosis in neurodegenerative disordersNat Rev Mol Cell Biol 2000 1(2):120-28.10.1038/3504000911253364 [Google Scholar] [CrossRef] [PubMed]
[25]. Mattson MP, Chan SL, Duan W, Modification of brain aging and neurodegenerative disorders by genes, diet, and behaviorPhysiol Rev 2002 82(3):637-72.10.1152/physrev.00004.200212087131 [Google Scholar] [CrossRef] [PubMed]
[26]. Darmon N, Pélissier MA, Heyman M, Albrecht R, Desjeux JF, Oxidative stress may contribute to the intestinal dysfunction of weanling rats fed a low protein dietJ Nutr 1993 123(6):1068-75. [Google Scholar]
[27]. Jalili C, Sohrabi M, Jalili F, Salahshoor MR, Assessment of thymoquinone effects on apoptotic and oxidative damage induced by morphine in mice heartCell MolBiol 2018 64(9):33-38.10.14715/cmb/2018.64.9.530030951 [Google Scholar] [CrossRef] [PubMed]
[28]. Andrade JP, Madeira MD, Paula-Barbosa MM, Differential vulnerability of the subiculum and entorhinal cortex of the adult rat to prolonged protein deprivationHippocampus 1998 8(1):33-47.10.1002/(SICI)1098-1063(1998)8:1<33::AID-HIPO4>3.0.CO;2-8 [Google Scholar] [CrossRef]
[29]. Robinson TE, Kolb B, Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaineEur JNeurosci 1999 11(5):1598-604.10.1046/j.1460-9568.1999.00576.x10215912 [Google Scholar] [CrossRef] [PubMed]
[30]. Dabydeen L, Thomas JE, Aston TJ, Hartley H, Sinha SK, Eyre JA, High-energy and-protein diet increases brain and corticospinal tract growth in term and preterm infants after perinatal brain injuryPediatrics 2008 121(1):148-56.10.1542/peds.2007-126718166569 [Google Scholar] [CrossRef] [PubMed]
[31]. Radak Z, Zhao Z, Goto S, Koltai E, Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNAMol Aspects Med 2011 32(4-6):305-15.10.1016/j.mam.2011.10.01022020115 [Google Scholar] [CrossRef] [PubMed]
[32]. Tuttle KR, Anderberg RJ, Cooney SK, Meek RL, Oxidative stress mediates protein kinase C activation and advanced glycation end product formation in a mesangial cell model of diabetes and high protein dietAm J Nephrol 2009 29(3):171-80.10.1159/00015447018781061 [Google Scholar] [CrossRef] [PubMed]
[33]. Biswas P, Mukhopadhyay A, Kabir SN, Mukhopadhyay PK, High-protein diet ameliorates arsenic-induced oxidative stress and antagonizes uterine apoptosis in ratsBiol Trace Elem Res 2019 5:01-02.10.1007/s12011-019-1657-230723882 [Google Scholar] [CrossRef] [PubMed]
[34]. Sarfert KS, Knabe ML, Gunawansa NS, Blythe SN, Western-style diet induces object recognition deficits and alters complexity of dendritic arborization in the hippocampus and entorhinal cortex of male ratsNutr Neurosci 2019 22(5):344-453.10.1080/1028415X.2017.138855729039252 [Google Scholar] [CrossRef] [PubMed]
[35]. Picciotto MR, Zoli M, Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s diseaseFront Biosci 2008 13(2):492-504.10.2741/269517981563 [Google Scholar] [CrossRef] [PubMed]
[36]. Giniatullin R, Nistri A, Yakel JL, Desensitization of nicotinic ACh receptors: shaping cholinergic signalingTrends Neurosci 2005 28(7):371-78.10.1016/j.tins.2005.04.00915979501 [Google Scholar] [CrossRef] [PubMed]
[37]. Vairapandi M, Balliet AG, Hoffman B, Liebermann DA, GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stressJ Cell Physiol 2002 192(3):327-38.10.1002/jcp.1014012124778 [Google Scholar] [CrossRef] [PubMed]
[38]. Żebrowska E, Maciejczyk M, Żendzian-Piotrowska M, Zalewska A, Chabowski A, High protein diet induces oxidative stress in rat cerebral cortex and hypothalamusInt J MolSci 2019 20(7):154710.3390/ijms2007154730925663 [Google Scholar] [CrossRef] [PubMed]
[39]. Mansouri MT, Naghizadeh B, Ghorbanzadeh B, Alboghobeish S, Amirgholami N, Houshmand G, Venlafaxine prevents morphine antinociceptive tolerance: The role of neuroinflammation and the l-arginine-nitric oxide pathwayExpNeurol 2018 303:134-41.10.1016/j.expneurol.2018.02.00929453978 [Google Scholar] [CrossRef] [PubMed]
[40]. Senaphan K, Sangartit W, Pakdeechote P, Kukongviriyapan V, Pannangpetch P, Thawornchinsombut S, Rice bran protein hydrolysates reduce arterial stiffening, vascularremodeling and oxidative stress in rats fed a high-carbohydrate and high-fat dietEur J Nutr 2018 57(1):219-30.10.1007/s00394-016-1311-027660232 [Google Scholar] [CrossRef] [PubMed]
[41]. Li C, Lesuisse J, Schallier S, Lamberigts C, Wang Y, Driessen B, The learning ability and memory retention of broiler breeders: 1 effects of reduced balanced protein diet on reward based learningAnimal 2019 13(6):1252-59.10.1017/S175173111800243430296963 [Google Scholar] [CrossRef] [PubMed]
[42]. Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning abilityExpNeurol 2008 211(1):141-49.10.1016/j.expneurol.2008.01.01618342310 [Google Scholar] [CrossRef] [PubMed]
[43]. Salahshoor MR, Roshankhah S, Motavalian V, Jalili C, Effect of harmine on nicotine induced kidney dysfunction in male miceInt J Prev Med 2019 10(1):97-104.10.4103/ijpvm.IJPVM_85_1831360344 [Google Scholar] [CrossRef] [PubMed]
[44]. Reyes-Castro LA, Padilla-Gómez E, Parga-Martínez NJ, Castro-Rodríguez DC, Quirarte GL, Díaz-Cintra S, Hippocampal mechanisms in impaired spatial learning and memory in male offspring of rats fed a low-protein isocaloric diet in pregnancy and/or lactationHippocampus 2018 28(1):18-30.10.1002/hipo.2279828843045 [Google Scholar] [CrossRef] [PubMed]
[45]. Nanou E, Catterall WA, Calcium channels, synaptic plasticity, and neuropsychiatric diseaseNeuron 2018 98(3):466-81.10.1016/j.neuron.2018.03.01729723500 [Google Scholar] [CrossRef] [PubMed]
[46]. Leclerc E, Trevizol AP, Grigolon RB, Subramaniapillai M, McIntyre RS, Brietzke E, The effect of caloric restriction on working memory in healthy non-obese adultsCNS Spectr 2019 10:01-07.10.1017/S109285291800156630968820 [Google Scholar] [CrossRef] [PubMed]