Fluoroquinolones belong to a series of synthetic antibiotics derived from quinolones, which are widely used at present to manage bacterial infections especially gram-negative bacteria. The same structure and mechanism of action have been further reported for all the compounds in the antibiotic quinolone group, which can be derived from the 4-aminoquinoline core and are also able to inhibit genome replication machine in sensitive bacteria in a selectively and irreversible manner [1,2].
Two strands of DNA are interconnected, but they need to be separated from each other during replication and transcription. For this purpose, the DNA gyrase enzyme make separate and reconnect strands. DNA gyrase is also recognised as a type II topoisomerase consisting of two subunits of A and B. Moreover, fluoroquinolones inhibit subunit A from DNA gyrase and induce the formation of a relaxation complex analog, which can prevent DNA twist during replication. These compounds can also cause bacterial death through inhibition of DNA replication and transcription [3,4]. Therefore, DNA gyrase is assumed to be essential for survival and proliferation of gram-positive and gram-negative bacteria, but not found in higher eukaryotes. Accordingly, this enzyme has become an attractive target for the design of antibacterial drugs.
Quinolones with a fluorine element in their core are called fluoroquinolones. In this respect; norfloxacin, and shortly thereafter, ciprofloxacin was among the first synthetic members of this group. The mechanism of action of fluoroquinolones is also similar to the quinolones, and their bactericidal properties depend on antibiotic concentrations. Moreover, fluoroquinolones are successful examples of anti-topoisomerase drugs with a broad spectrum effects . However, in recent years, due to the spread of antibiotic resistance, gram-negative and gram-positive bacteria have become progressively more resistant to fluoroquinolones.
Since resistance to fluoroquinolones usually arises by changes in the target enzyme, DNA-gyrase, it is necessary to overcome this antibiotic resistance by altering primary fluoroquinolones or producing their derivatives via the inhibitory effect on resistant mutants [5-7].
Substituting fluorine at the sixth position is also considered as the differential domain of the fluoroquinolones with quinolones and the reason for increasing antimicrobial effects on both gram-negative and gram-positive bacteria. Fluorination also improves drug penetration into bacterial cells and increases drug efficacy [8]. Adding the cyclopropyl group at the first position (enrofloxacin and ciprofloxacin), ethyl or fluorophenyl can thus develop an antibacterial spectrum on gram-negative and gram-positive bacteria. Adding piperazine at the seventh position also improves the effect spectrum of P. aeruginosa and other gram-negative bacteria. Changes in the nitrogen group to the carbon group at the eighth position also reduce the negative effects on the central nervous system and consequently increase antibacterial effects on P. aeruginosa [9]. Therefore, any alterations, even brief ones, in this structure, as well as substitution of different groups, will be assumed responsible for the development of various physiological effects including differences in lipophilicity, tissue release rates, oral intake, as well as excretion rates. It should be noted that such changes do not transform the antibacterial effect spectrum e.g., enrofloxacin has a fluorine unit, while difloxacin contains two groups of fluorine, and orbifloxacin involves three fluorine groups. Nevertheless, antibacterial activity does not increase in all of these derivatives as the number of fluorine units are added. To the best of our knowledge, no studies had been conducted hitherto to attribute these differences to various clinical responses although some of these structural differences can affect absorption and distribution of antibiotics in the body. For example, oral absorption of ciprofloxacin is approximately half of that in enrofloxacin [9].
Therefore, the present study was conducted to investigate the inhibitory effect of 5a and 5b synthesised in our previous research [10], using disk diffusion test and broth microdilution method, and to explore their effects on DNA gyrase gene expression levels in E. coli and P. aeruginosa compared with gentamycin (GM 10 μg/disc) and ciprofloxacin (CP 5 μg/disc), both as positive controls.
Materials and Methods
An in vitro experimental study was conducted during March 2017 till February 2018 to evaluate two derivatives of fluoroquinolones, 5a and 5b, compounds which were synthesised in the Chemistry Department of the Faculty of Sciences at the Vali-e-Asr University of Rafsanjan, Kerman, Iran, and tested for their antimicrobial property on E. coli and P. aeruginosa in the Rafsanjan University of Medical Sciences, Kerman, Iran. The study was done under institutional ethic code No: IR.RUMS.REC.1395.139.
Synthesis of Compound 5a and 5b
Synthesis of 5a (N-4-methyl (phenyl)-2,2,2- trifluoroacetimidoyl ciprofloxacin)
We added 0.33g (0.001 mol) of ciprofloxacin (Sigma, the United States), 10 mL of N, N-dimethylformamide (Merck, Germany) and 0.27g (0.001 mol) of potassium carbonate (Merck, Germany) to a 250-mL flask and then stirred for 10 minutes at the ambient temperature to achieve a homogenous mixture. Then, 0.31 g (0.001 mol) of 2,2,2-trifluoro-N-(p-tolyl)acetimidoylchloride (Merck, Germany), was dissolved in 15 mL of dimethylformamide, poured dropwise into the flask, refluxed for 26 hours. After completion of the reaction, the solvent was removed via rotary evaporation and the resulting product was purified with dimethylformamide. Next, it was washed with ether solvent. Finally, a cream-colored and pure product was obtained. (melting point: 206°C) [Table/Fig-1] [10].
Synthesis of Compounds 5a (N-4-methyl (phenyl)-2,2,2- trifluoroacetimidoyl ciprofloxacin) and 5b (N-4- methyl (phenyl)-2,2,2-trifluoroacetimidoyl norfloxacin).
1; 4-methylaniline, 2; trifluoroacetic acid, 3; 2,2,2-trifluoro-N-(p-tolyl)acetimidoylchloride,. 4a, 4b; molecular base of ciprofloxacin or norfloxacin depending on kind of R
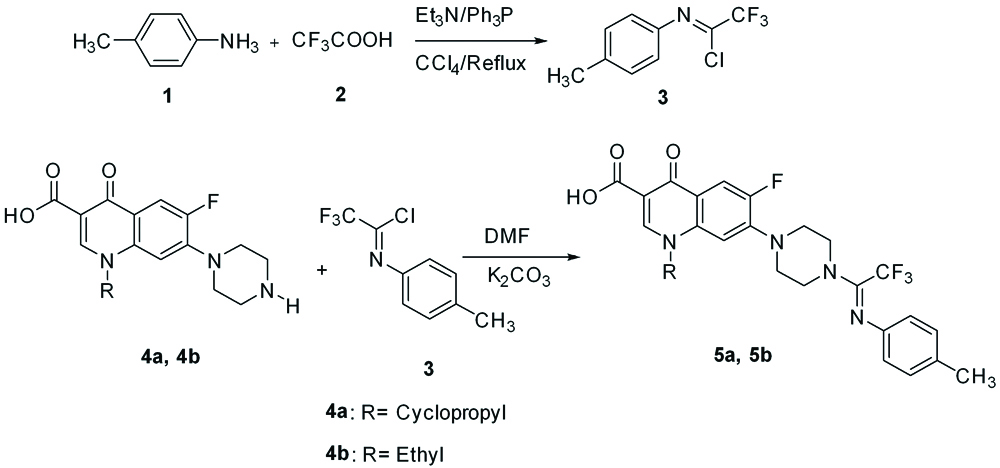
Synthesis of 5b (N-4- methyl (phenyl)-2,2,2-trifluoroacetimidoyl norfloxacin)
We added 0.33g (0.001 mol) of norfloxacin (Sigma, the United States), 10 mL of N, N-dimethylformamide, and 0.27 g (0.001 mol) of potassium carbonate to a 250-mL flask, subsequently stirred for 10 minutes at the ambient temperature to become uniform, followed by dissolution of 0.31 g (0.001 mol) of 2,2,2-trifluoro-N-(p-tolyl)acetimidoyl chloride, in 15 mL of dimethylformamide, and dropwise addition to the flask, refluxed for 24 hours. After completion of the reaction, the solvent was removed via rotary evaporation and the resulting product was purified with dimethylformamide. Next, it was washed with ether solvent. Finally, the milky sedimentary and pure product was obtained. (Melting Point: 214°C) [Table/Fig-1] [10,11].
Bacterial Cultivation and Verification
E. coli and P. aeruginosa, respectively with PTCC 1399 (ATCC 25922) and PTCC 1707 (ATCC 15442), were purchased from the Center for Persian Type Culture Collection and exited from the lyophilized state through adding 0.9% sodium chloride (normal saline) according to the manufacturer’s instructions [12,13]. To ensure viability and quality, the bacteria were cultured in Blood agar (BA) medium (Merck, Germany) and verified again using phenotypic and biochemical tests. Fresh cultures and pure colonies were finally obtained from each of the bacteria.
Disc Diffusion Test
Antibiotic susceptibility testing was performed for 5a and 5b compounds as well as ciprofloxacin and gentamycin antibiotics (as the positive controls) using Kirby-Bauer disc diffusion test in accordance with the guidelines of the Clinical and Laboratory Standards Institute (CLSI) for both bacteria in this study. To increase the statistical accuracy of the results, the experiments were repeated three times [14,15].
Susceptibility testing of 5a, 5b, ciprofloxacin, and gentamycin using broth microdilution method
The MIC values of 5a, 5b, ciprofloxacin, and gentamycin were determined using the broth microdilution method on the basis of the CLSI guidelines for both bacteria in this study. Initially, 1024 mg of antibiotics (ciprofloxacin and gentamycin) and fluoroquinolone derivatives (compound 5a and 5b) were seriated to provide concentrations of 2-512 μg/mL. Then, a constant amount of bacteria (equal to 0.5 McFarland) was cultured onto Muller hinton broth (MHB) medium in the presence of serial dilutions of the tested compounds. After incubation for 24 hours at 37°C, the MIC values were determined for the given compounds and antibiotics [16,17].
Evaluation of DNA gyrase gene expression levels in the presence of 5a and 5b compounds
At first, fresh bacterial cultures were prepared on BA medium. Then, the appeared colonies were used for inoculation in Tryptic soy broth (TSB). Afterward, the TSB was treated by the 1.2×MIC concentration of 5a and 5b separately and placed in a shaking incubator with 180 rpm at 37°C. After reaching logarithmic growth phase (the Optical density (OD) of 0.6 at a wavelength of 650 nm), the whole messenger RNA (mRNA) was extracted from the broth culture media containing bacteria treated with 5a and 5b using an mRNA extraction kit (Cat.# 9767, Takara, Kyoto, Japan). Briefly; lysis suspension bacteria were centrifuged at 8,000 Xg at 4°C for 2 minutes, then the supernatant was discarded and the cell pellet was re-suspended with 1X Phosphate-Buffered Saline (PBS), and subsequently centrifuged at 8,000 rpm at 4°C for 2 minutes again. An appropriate volume of the RL (RNA lysis) buffer was also added to the cell pellets and the cell lysates were incubated at room temperature for 2 minutes. Next, the lysates were applied to genomic DNA (gDNA) Eraser Spin Column in the collection tube and subsequently centrifuged at 12,000 rpm for 1 minute. The gDNA Eraser Spin Column was also discarded and an equal volume of 70% ethanol was added to the flow-through in the 2 mL collection tube and mixed thoroughly by pipetting it up and down. Furthermore, the mixture was applied to RNA Spin Column in a 2-mL collection tube and centrifuged at 12,000 rpm for 1 minute. The flow-through was similarly discarded and 500 μL Risk-Weighted Asset (RWA) buffer was added to RNA Spin Column and successively centrifuged at 12,000 rpm for 30 seconds and the flow-through was discarded (this step was repeated). Finally, 350 μL of RWA buffer was added to the RNA Spin Column and centrifuged at 12,000 rpm for 30 seconds.
The complementary DNA (cDNA) was also generated from the yielded total mRNA, employing PrimeScript™ II 1st strand cDNA Synthesis Kit (TaKaRa Bio, Shiga, Japan). The RT-PCR was subsequently performed in a 20 μL solution containing 10 μL SYBR® Premix Ex Taq TM (Takara, Japan), 0.2 μL ROX, 1.5 μL of normalised cDNA, and 1 μM of each primer, specific for DNA gyrase and 16s and up to 20 μL nuclease-free water. The specific primers of DNA topoisomerase IV (DNA gyrase) subunit A (Pseudomonas aeruginosa ATCC 15442) forward: ACGTACTAGGCAATGACTGG; reverse: AGAAGTCGCCGTCGATAGAAC, DNA topoisomerase IV (DNA gyrase) subunit A (E. coli ATCC 25922) forward: 5′-CGCGGATCCCGCCGTGAGTACCACCG; reverse: CGCGGATCCCTGCCCCGTCTGCTC as the target gene and Universal 16sRNA forward: CGGTCCAGACTCCTACGGGAGGCAGCA; reverse: GCGTGGACTACCAGGGTATCTAATCC as a reference gene were also used. Each separate RT-PCR assay was completed in triplicate using a thermal cycler ABI Step One Plus® system (Applied Biosystems™, the US). Whereas, 16s was considered as a house-keeping gene (reference gene) aimed at normalisation of the amplified signals of the DNA gyrase gene. Additionally, the relative DNA gyrase levels were calculated by 2-∆∆Ct wherein ∆Ct=Ct (DNA gyrase)-Ct (16s) and ∆∆Ct=∆Ct (treated E. coli and P. aeruginosa with 5a and 5b)-∆Ct (untreated E. coli and P. aeruginosa) [18].
Statistical Analysis
Data collected from the disc diffusion test and broth microdilution method as well as analysis of DNA gyrase gene expression levels using RT-PCR, were inserted into SPSS Statistics (version 21) software. The results of the mean diameter of the ZOI from each of 5a and 5b compounds in the dilutions of 1:2, 1:4, 1:8, 1:16, 1:32, and 1:64 to 1:512 prepared from primary concentration (5 mg/mL) were then evaluated and compared with the bacteria in this study. The data were further analysed using “one-way ANOVA” and significant results using “Tukey’s multiple comparison tests”. In addition, the results of the statistical analysis were reported as “mean±standard” deviation at the significance level of 0.05.
Results
Effect of Growth Inhibitor Compounds Evaluated by Disc Diffusion Test
The difference between the mean ZOI diameters for P. aeruginosa exposed to 5a (18.5±0.1 mm) and ciprofloxacin (28.0±2.0) was not statistically significant (p>0.05). Moreover, the difference between the mean ZOI diameters for E. coli in the presence of 5a (13.2±0.1 mm) and ciprofloxacin (18.3±0.1 mm) was not reported statistically significant (p≥0.05). Compared with 5a, ciprofloxacin was found more effective in inhibiting P. aeruginosa and E. coli (the ZOI diameter of ciprofloxacin was also higher than that of the 5a). The results from the inhibitory effect of 5b on the growth of P. aeruginosa (28.0±0.1 mm) compared with ciprofloxacin (28.0±2 mm) were not significant (p=0.99). The inhibitory effect of 5b on P. aeruginosa was approximately the same as that of ciprofloxacin, while the mean ZOI diameter of E. coli in the presence of 5b (38±0.1 mm) and ciprofloxacin (17.9±0.1 mm) was reported to be statistically significant (p<0.01), so that the ZOI diameter of 5b was twice as high as ciprofloxacin.
The mean ZOI diameter of P. aeruginosa in the presence of 5a (18.5±0.1 mm) and gentamycin (21±0.1 mm) was not statistically significant. Therefore, 5a had less efficacy against P. aeruginosa compared with gentamycin. Comparing the results of mean ZOI diameter of E. coli in the presence of the 5a (13.2±0.1 mm) and gentamycin (19.0±0.1 mm) the difference between them was not statistically significant (p=0.61). The inhibitory effect of 5b compared with gentamycin also showed that the mean ZOI diameter of P. aeruginosa was statistically significant in the presence of 5b (28.0±0.1) and gentamycin (21±0.1 mm) (p<0.01). Therefore, 5b had a greater inhibitory effect on P. aeruginosa. In addition, the mean ZOI diameter of E. coli in the presence of 5b and gentamycin were (38.0±0.1 mm) and (19.0±0.1 mm) respectively and it was statistically significant (p<0.01), indicating that 5b in used concentration was more effective than gentamycin against tested gram-negative bacteria [Table/Fig-2].
Inhibition zone caused by MIC microgram concentration of antibacterial candidates and some therapeutic antibiotics against Pseudomonas aeruginosa and Escherichia coli (Triple tests).
| Gram-negative bacteria | Compound | Drugs (μg) | Inhibition zone (mm) |
|---|
| Pseudomonas aeruginosa | 5a | 32 | 18.5 |
| 18.7 |
| 18.4 |
| 5b | 8 | 28 |
| 27.9 |
| 28 |
| GM | 10 | 21 |
| 21.1 |
| 21 |
| CP | 5 | 28 |
| 27.8 |
| 28.2 |
| Escherichia coli | 5a | 128 | 13.2 |
| 13.1 |
| 13.2 |
| 5b | 256 | 38 |
| 38 |
| 38.1 |
| GM | 10 | 19 |
| 19 |
| 19.1 |
| CP | 5 | 17.9 |
| 18.1 |
| 18.1 |
GM 10 mcg: Gentamycin (GM10); CP 5 mcg: Ciprofloxacin (CP5)
Effect of Growth Inhibitor Compounds 5a and 5b Evaluated by Broth Microdilution Method
The MIC value of 5a and gentamycin were 32 μg/mL and 64 μg/mL for P. aeruginosa respectively. The MIC value of ciprofloxacin in P. aeruginosa was also reported by 16 μg/mL. Moreover, the MIC value of 5b for P. aeruginosa was 8 μg/mL which had been reduced in comparison with gentamycin and ciprofloxacin by 8 and 2 folds, respectively. Besides, the results of the MIC value of 5b in P. aeruginosa were in line with the findings of the disc diffusion test, suggesting a greater inhibitory effect compared with gentamycin and ciprofloxacin.
The MIC value of 5a in E. coli was 128 μg/mL that was also 2 and 4 folds higher than that of gentamycin and ciprofloxacin (64 μg/mL, 32 μg/mL) respectively. The MIC value of 5b in E. coli was 256 μg/mL, indicating a reduction of 4 and 8 folds, (64 μg/mL, 32 μg/mL) respectively, compared with gentamycin and ciprofloxacin, confirming the results of the disc diffusion test. In general, the results of the MIC value of 5b in P. aeruginosa and E. coli were endowed with greater antimicrobial effects than gentamycin and ciprofloxacin, while 5a had no appropriate efficacy in comparison with the control antibiotics [Table/Fig-2,3].
The Minimum inhibitory concentration (MIC) of 5a and 5b compounds, as well as gentamycin and ciprofloxacin.
| Compounds | MIC (μg/mL) |
|---|
| Pseudomonas aeruginosa | Escherichia coli |
|---|
| Gentamycin | 64 | 64 |
| Ciprofloxacin | 16 | 32 |
| 5a | 32 | 128 |
| 5b | 8 | 256 |
Effects of 5a and 5b compounds as well as gentamycin and ciprofloxacin antibiotics on DNA Gyrase gene expression levels
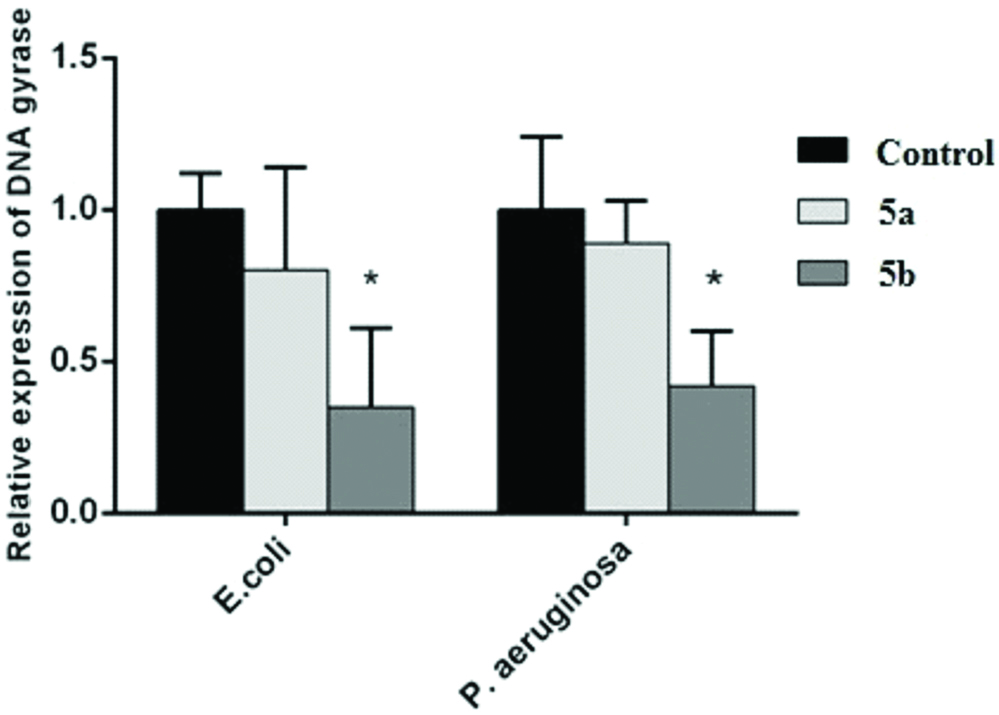
The results of the analysis of the gene expression using RT-PCR showed that the DNA gyrase gene expression levels had reduced following the addition of ciprofloxacin and gentamycin compared with the antibiotic-free sample. The DNA gyrase gene expression levels in both gram-negative bacteria also decreased after treatment with 5a and 5b but this descending trend was only statistically significant for 5b in comparison with the control sample (the antibiotic-free medium) [Table/Fig-4], which was similar to that of disc diffusion test and MIC.
Illustrates alteration in the gene expression of specific DNA gyrase gene after treatment with 5a and 5b compared to the control. The relative DNA gyrase levels were calculated by 2-∆∆Ct wherein ∆Ct=Ct (DNA gyrase)-Ct (16s) and ∆∆Ct=∆Ct (treated E. coli and P. aeruginosa with 5a and 5b)-∆Ct (untreated E. coli and P. aeruginosa).
*significant difference, p<0.05
Discussion
The present study examined the antibacterial effect of synthetic 5a and 5b compounds as alternative candidates for fluoroquinolones against E. coli PTCC 1399 (ATCC 25922) and P. aeruginosa PTCC 1707 (ATCC 15442) using disc diffusion test and broth microdilution method and also analysed the impact of these compounds on DNA gyrase gene expression levels compared with ciprofloxacin and gentamycin. The results also revealed that the mean ZOI diameter of E. coli and P. aeruginosa in the presence of 5a was not statistically significant compared with ciprofloxacin and gentamycin, and these compounds had not the required efficacy compared with ciprofloxacin and gentamycin against gram-negative bacteria. According to the findings, the mean ZOI diameter of 5b in comparison with gentamycin showed better antibacterial effects against E. coli and P. aeruginosa and this effect was much higher in case of E. coli but compared with ciprofloxacin in E. coli and P. aeruginosa had the same effect as ciprofloxacin. 5b in the MIC value also showed similar results to those obtained by disc diffusion test and severely reduced the MIC value for both bacteria. Moreover, 5b revealed good effects in comparison with both antibiotics, while this effect was not observed for 5a. The results of the expression level of DNA gyrase gene after treatment with 5a and 5b compounds exhibited that both compounds had reduced gene expression at the RNA level in E. coli and P. aeruginosa, but the reduction in 5b was statistically significant, confirming the results obtained from disc diffusion test and MIC. After treating the bacteria with 5b, the DNA gyrase gene expression levels had also decreased by more than half compared with the control sample. Although reduced expression was observed in 5a, this descending trend was not significant and 5a showed an inadequate antibacterial effect compared with the two antibiotics used.
Evidence from E. coli strains isolated from hospital-acquired infections suggested that taking ciprofloxacin could reduce bacterial sensitivity of the drug and this could make resistant bacteria unable to be treated. This could be a hazard for the treatment of infections caused by this bacterium and highlighted the importance of synthesising new fluoroquinolone-derived compounds [19]. The effect of high concentrations of ciprofloxacin against P. aeruginosa was also examined in a study and it was reported that increasing drug concentration to 2-4 folds could prevent growth and, consequently, development of resistance in this bacterium [12]. However, this approach could have numerous side effects such as headache, dizziness, swelling, nausea, vomiting, stomach upset, bloody or watery diarrhea, etc., [20]. Therefore, new compounds were endowed with acceptable antimicrobial effects and also proper applications. Hence, the derivatives of fluoroquinolones, as well as the compounds in this study, could be a more appropriate strategy for the use of antimicrobial compounds [21].
Our previous study, Darehkordi A et al., showed that both ciprofloxacin and norfloxacin derivatives had a positive antibacterial effect on some gram negative and gram positive bacteria (Escherichia coli, Klebsiella pneumoniae and S. aureus), but we didn’t test the MICs and expression of involved gene in those bacterial strains [10], while in case of the other study, Darehkordi A et al., focused on Synthesis of new indole trifluoromethyl derivatives and successfully synthesised a new series of indole trifluoromethyl derivatives using different 2,2,2-trifluoro-narylacetimidoyl chlorides. Its efficiency, simplicity and light reaction condition allows high production of trifluoromethyl indole [11].
In our previous study, N-substituted-trifluoroacetimidoyl chlorides were used to synthesise piperazinyl quinolone derivatives. Two derivatives obtained in this way were considered to determine antibacterial activity and to compare them with antibiotics available in the market, including ciprofloxacin and norfloxacin, using disc diffusion test against various bacteria such as E. coli vs. the controls in a way that some of these compounds showed good antibacterial effects [10]. In the present study, 5b had a greater effect on ciprofloxacin concentration against the growth of E. coli and P. aeruginosa. Therefore; it was concluded that 5b can be used as a tailored substitute for treatment of infections caused by E. coli and P. aeruginosa, based on further studies including experiments on animal models and then human volunteers. The important point in the use of 5b is the different behavior of the two tested bacteria in terms of their efficacy on the compound, which could be attributed to differences in physiological characteristics of two bacterial genera. It should be noted that even two sensitive strains to the same drug, isolated from a specific patient with susceptibility to the antimicrobial agent (as control) and increased ZOI, can still be an advisable drug for the treatment of the corresponding infection, despite the impressible severity different from an antimicrobial compound.
Contrary to the results of this study, Foroumadi A et al., showed that new quinolone derivatives from phenylethyl ether gatifloxacin resulting from substitution at the seventh position of quinolone by BA dilution had significant antimicrobial effects on gram-positive bacteria but could not evaluate these impacts on gram-negative ones [22]. In this respect, Emami S et al., used 7-piperazinyl core to synthesise quinolones containing methylated nitrofuran, which had an antibacterial effect. Once the ethyl group and Carbon-Fluorine (CF) were used, respectively, at N-1 and C-8 positions, the compounds induced a better antibacterial effect on E. coli and Bacillus subtilis [23].
The effect of 5a (adding the ethyl group to the base molecule of ciprofloxacin base molecule) on E. coli and P. aeruginosa growth was different from that of 5b (adding cyclopropyl group to base molecule of norfloxacin), gentamycin, and ciprofloxacin, which could be due to differences in the molecular structure of the antimicrobial compound, physiological conditions of the tested bacteria, and others. It seems to add ethyl group to norfloxacin base molecule has better efficiency than the cyclopropyl group. For this reason, it may act better in compare to 5a, gentamycin, and ciprofloxacin. Therefore, adding ethyl group to norfloxacin base molecule has better efficiency than cyclopropyl group. Another study, Malík I et al., also showed that substitution of one or more groups including fluorine could increase the antimicrobial effects of the drug or even act in the opposite direction and consequently reduce the antimicrobial effect of the base compound [24].
In recent years, changes in the structure of the new quinolone compounds have been always a matter of concern for researchers. For example; in line with the results of the present study, a new fluoroquinolone of WQ-3810 and another compound synthesised via reducing amination of fluoroquinolones and having antibacterial activity on DNA gyrase of E. coli was reported to have the requisite qualities to be used for treatments. Findings of this study, Dixit SK et al., demonstrated significant success in the synthesis and use of new compounds derived from fluoroquinolones [25].
It was concluded that synthesised and evaluated compounds in the present study might not be suitable as an exact alternative to ciprofloxacin in managing infections caused by P. aeruginosa. Anyway, 5b as a new derivative of fluoroquinolone compared with gentamycin had an antibacterial effect against tested gram-negative bacteria especially P. aeruginosa at phenotypic and molecular levels. Also, the antibacterial activity of 5b against E. coli in the used concentration represented more ZOI diameter in compare to gentamycin and ciprofloxacin.
Limitation
Some limitation of our present study is; few bacteria and, only two fluoroquinolone derivatives were studied. It was better to perform the study with ciprofloxacin and gentamycin-resistant bacteria to see whether the tested fluoroquinolone derivatives could inhibit the growth of ciprofloxacin and gentamycin Pseudomonas aeruginosa and E. coli or not.
Conclusion
Compound 5b as a new derivative of fluoroquinolone compared with gentamycin, had an antibacterial effect against tested gram-negative bacteria especially P. aeruginosa at phenotypic and molecular levels, and it could also be an appropriate candidate for alternative drugs. Further studies on other existing or ongoing fluoroquinolone-resistant gram-negative bacteria may result in comprehensive findings in terms of the use of this compound in treatments of infections caused by gram-negative bacteria. Since the resistance to common antibiotics can have adverse effects on the treatment of bacterial infections, it is essential to discover or synthesise new antibacterial agents.