Lymphatic vessel invasion in colorectal cancer is reported to be an important risk factor correlated with poor prognosis [5]. Evaluation of lymphovascular invasion helps in determining the high risk patients in the same TNM stage and the possibe need of adjuvant chemotherapy [6,7]. Podoplanin (D2-40) monoclonal antibody is a transmembrane sialoglycoprotein that is expressed specifically in lymphatic endothelial cells only. It does not exist in the endothelial cells of blood vessels, so it can discriminates between lymphatic and blood vessel [8-10].
Thus, the aim of this study was to assess PELI and LVI in colorectal carcinoma cases and determine their role in evaluating pT3 CRC and correlating them with tumour prognostic parameters and pT4a tumours.
Materials and Methods
This retrospective study included 60 cases of CRC {pT3 (40 cases) and pT4a (20 cases)} obtained from the Pathology Department, Faculty of Medicine, Cairo University during the period from January 2016 until September 2017. Case selection for pT3 CRC were confined to paraffin blocks enclosing the deepest tumour wall invasion; partly or totally covered with the peritoneum. Cases with incomplete clinical data were excluded.
Sections were re-cut from all chosen blocks and stained with Haematoxylin and eosin (H&E). They were examined and classified according to the recommendations of the World Health Organisation (2010) [11] including histological types, depth of tumour invasion, perineural invasion, lymphovascular emboli and number of lymph nodes harvested. The TNM staging was applied on each case according to the American Joint Committee of Cancer (AJCC) and the International Union for Cancer Control (UICC) where it encodes the extent of the primary tumour (T), regional lymph nodes (N), and distant metastases (M) [12].
Verhoeff’s Staining Procedure
Sections of 4 microns thick were prepared from blocks of pT3 cases and mounted on glass slides and stained by Verhoeff’s stain [13], to detect peritoneal elastic lamina invasion and the elastic layer of the blood vessels. The pT4a cases were not stained as the tumour cells were already penetrating the serosal surface in all pT4a cases.
Assessment of peritoneal elastic lamina staining was performed. The elastic fibers in the peritoneal elastic lamina were stained black. Verhoeff’s staining of elastic lamina of the colonic mural vessels served as internal control. The elastic lamina of the extramural blood vessels presents beyond the muscularispropria; near the primary colorectal tumour; was also stained by Verhoeff’s stain. This facilitates the detection of tumour Extramural vascular invasion (EMVI).
Classification of pT3 cases according to verhoeff’s staining: Negative PELI (no invasion); Positive PELI: (with invasion); PELI unidentified. |In cases where the peritoneal elastic lamina was disturbed by the tumour-associated inflammation or desmoplastic reaction but not by the tumour itself, only those showing tumour invasion beyond a line drawn between the remaining elastic lamina were considered positive for PEL [3].
Immunohistochemical Study Procedure
A serial section (4 μM) from all cases was mounted on adhesive-coated glass slides for Podoplanin (D2-40) staining to detect lymphovascular invasion. Primary antibody included is mouse monoclonal (D2-40) antibody to Podoplanin/gp36 (Abcam; ab77854, Cambridge, MA), at a 1/40 dilution. The Biotin based DAKO Envision system (DAKO Envision labelled polymer, peroxidase) was used as the detection system. The sections were incubated with the antibody for 60 minutes at room temperature. Internal negative control was endothelium of included colonic small blood vessels and positive control was the endothelium of lymphatic vessels of muscularispropria or of the subcapsular space and interstitial lymphatic vessels of omental lymph nodes of the colonic section. Topographic Assessment of podoplanin expression in endothelial cells of lymphatic vessels for detection of the lymphatic vessels in the colonic wall was performed and LVI was considered positive when at least one cancer cell was clearly visible inside a podoplanin positive endothelial vessel. Podoplanin expression was found also in nerve cells that facilitate the detection of perineural invasion by the tumour and in the mesothelial cells of the peritoneal layer covering the colonic wall.
Cases were also classified according to presence of lymphovascular invasion detected by D2-40 immunohistochemistry into cases with positive D2-40 detected LVI and negative D2-40 detected LVI.
Statistical Analysis
Microsoft Excel 2016 was used for data entry and the statistical package for social science (SPSS version 25) was used for data analysis.
Simple descriptive statistics (arithmetic mean and standard deviation) to summarise quantitative data and frequencies are used for qualitative data.
The bivariate relationship was displayed in cross-tabulations and comparison of proportions was performed using the chi-square test.
The t-independent test was used to compare normally distributed quantitative data.
All p-values are two-sided. The p-values <0.05 were considered significant.
Results
This retrospective study was conducted on sixty cases of pT3 and pT4a colorectal cancer. The age of the studied cohort ranged from 20-81 years (mean±SD=50±14) and it included 37 females and 23 males.
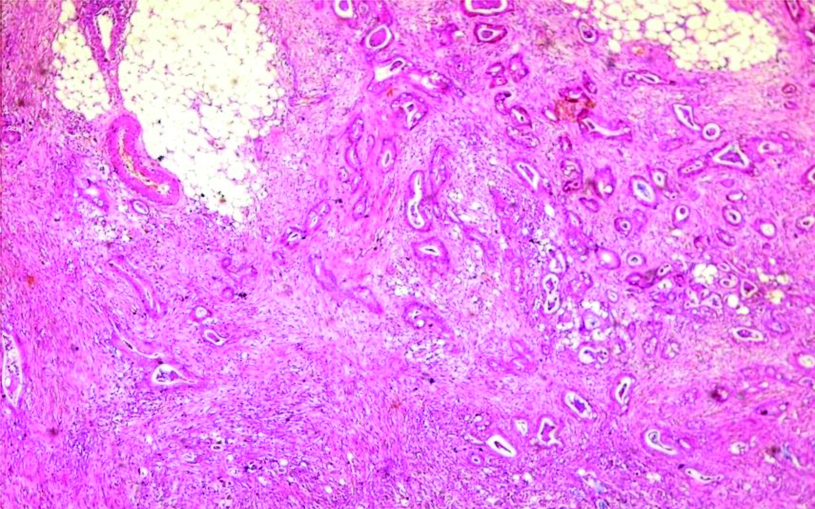
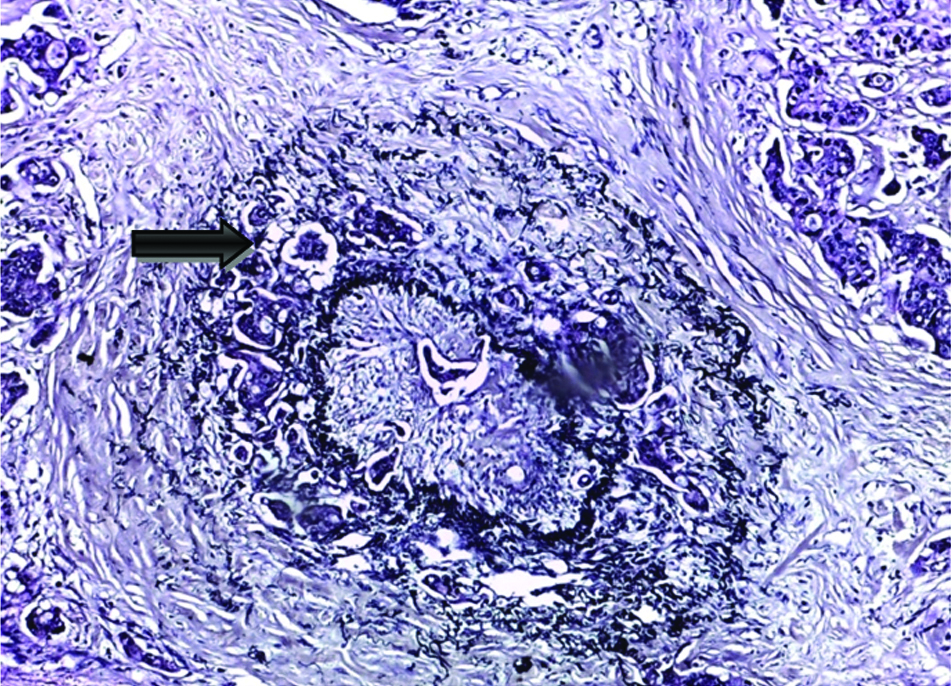
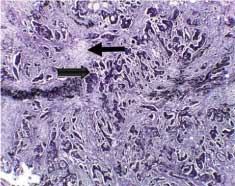
The majority of studied cases were either adenocarcinoma or adenocarcinoma with mucoid activity representing 50 cases (83.33%) [Table/Fig-1]. Only 2 cases showed extramural vascular invasion, as evidenced by vascular elastic lamina staining [Table/Fig-2].
Colonic adenocarcinoma, shows irregular glands infiltrating subserosal fat (H&E, original magnification X100).
Extramural tumoural vascular invasion (arrow), the tumour was seen infiltrating the wall of the blood vessel that visualised by Verhoeff’s staining of the internal and external elastic lamina (original magnification X400).
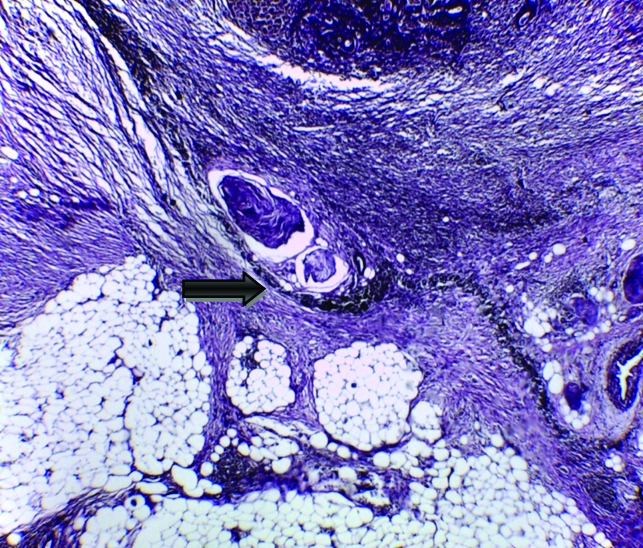
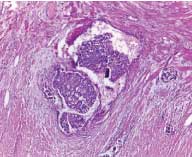
According to the status of peritoneal invasion, the studied cases were divided into 20 cases with negative PELI [Table/Fig-3], 16 cases with positive PELI [Table/Fig-4,5], 4 cases with PEL unidentified and 20 cases with peritoneal penetration (pT4a).
Negative peritoneal elastic lamina invasion (arrow), Verhoeff’s elastic staining (original magnification X400).
Positive peritoneal elastic lamina invasion (arrow) shows tumour invasion and desmoplastic stromal reaction as visualised by Verhoeff’s elastic staining (original magnification X100).
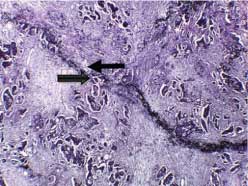
Positive peritoneal elastic lamina invasion (arrow) shows tumour invasion and desmoplastic stromal reaction as visualised by Verhoeff’s elastic staining (original magnification X400).
In T3 CRC, positive PELI status had a statistically significant in relations with distant metastasis, TNM stage, lymph node metastasis, LVI and D2-40 detected LVI. Meanwhile, it showed no statistically significant correlation with age & sex of patients, site of the primary tumour, tumour maximal diameter, gross pattern, the tumour histologic type, the presence of necrosis (data not shown), perineural invasion, peritoneal nodules and EMVI [Table/Fig-6].
Clinicopathological characteristics of the studied pT3 CRC cases and its correlation with PELI status.
| Clinicopathologic features | T3 CRC cases (n=40, 100%) | Total (n=40, 100%) | p-value |
|---|
| PELI-ve | PELI+ve | PELI undetected |
|---|
| (n=20, 50%) | (n=16, 40%) | (n=4, 10%) |
|---|
| Peritoneal nodules |
| Absent | 18 (90%) | 13 (81.25%) | 3 (75%) | 34 (85%) | 0.643 |
| Present | 2 (10%) | 3 (18.75%) | 1 (25%) | 6 (15%) | |
| >EMVI |
| Absent | 19 (95%) | 15 (93.75%) | 4 (100%) | 38 (95%) | 0.877 |
| Present | 1 (5%) | 1 (6.25%) | 0 (0%) | 2 (5%) | |
| >LVI |
| Absent | 14 (70%) | 5 (31.25%) | 0 (0%) | 19 (47.5%) | 0.009 |
| Persent | 6 (30%) | 11 (68.75%) | 4 (100%) | 21 (52.5%) | |
| Perineural invasion |
| Absent | 13 (65%) | 7 (43.75%) | 2 (50%) | 22 (55%) | 0.435 |
| Persent | 7 (35%) | 9 (56.25%) | 2 (50%) | 18 (45%) | |
| LN metastasis |
| Absent | 13 (65%) | 0 (0%) | 2 (50%) | 15 (37.5%) | 0.001 |
| Present | 7 (35%) | 16 (100%) | 2 (50%) | 25 (62.5%) | |
| Distant metastasis |
| Absent | 20 (100%) | 12 (75%) | 4 (100%) | 36 (90%) | 0.036 |
| Present | 0 (0%) | 4 (25%) | 0 (0%) | 4 (10%) | |
| TNM stage |
| Stage II | 12 (60%) | 1 (6.25%) | 2 (50%) | 15 (37.5%) | |
| Stage III | 8 (40%) | 11 (68.75%) | 2 (50%) | 21 (52.5%) | <0.001 |
| Stage IV | 0 (0%) | 4 (25%) | 0 (0%) | 4 (10%) | |
| D2-40 expression of lymphatic vessels |
| Negative | 17 (85%) | 1(6.25%) | 2 (50%) | 20 (50%) | <0.001 |
| Positive | 3 (15%) | 15 (93.75%) | 2 (50%) | 20 (50%) | |
D2-40 increased the detection rate of angiolymphatic invasion by 16.7% (10 cases) over routine H&E [Table/Fig-7,8,9]. D2-40 expression in nerve bundles of the colonic wall fascilitates the detection of perineural tumour invasion in 24 cases [Table/Fig-10].
Peritumoural lymphovascular emboli in colonic adenocacinoma, H&E (original magnification X400).
Lymphovascular emboli visualised by Podoplanin (D2-40) immunohistochemically expression in the endothelial cells lining peritumoural lymphatic vessels in colonic adenocarcinoma (original magnification X400).
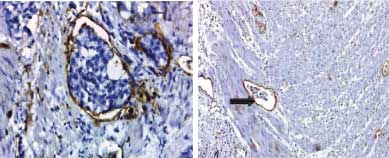
Perineural tumour invasion (arrow) in colonic adenocarcinoma, the nerve bundles were expressed by Podoplanin (D2-40) immunohistochemistry (original magnification X400).
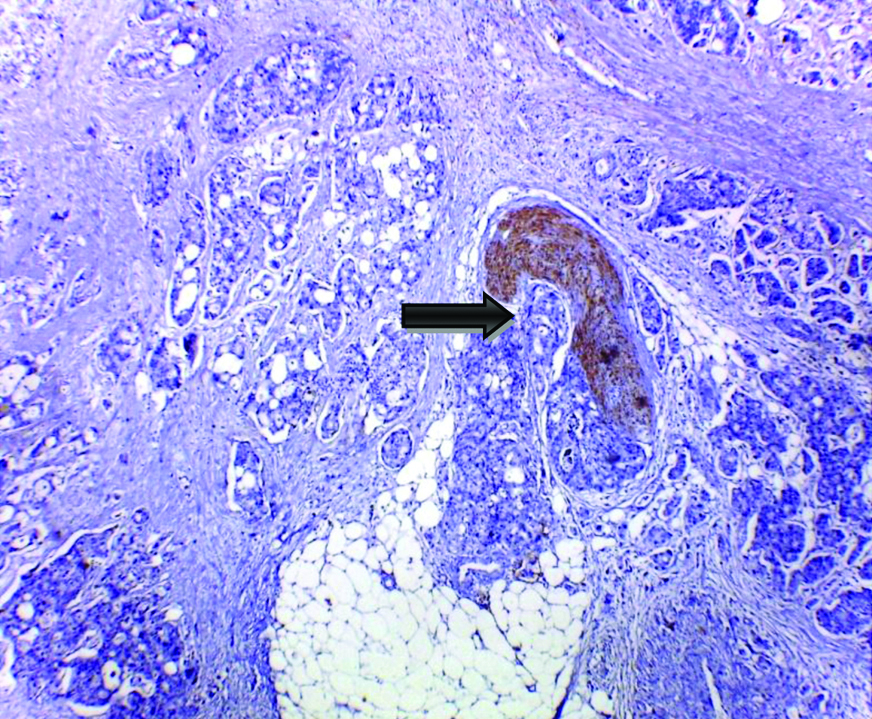
D2-40 immunohistochemically detected LVI was significantly correlated with lymph node metastasis, LVI, perineural invasion, peritoneal nodules and the TNM stage [Table/Fig-11]. Though no significant correlation between patients’ age, gender, tumour histologic type, extent of tumour, presence of necrosis (data not shown) and EMVI.
The correlation between D2-40 immunohistochemically identified LVI in studied CRC cases and clinicopathological features.
| Clinicopathologic features | D2-40 -ve | D2-40 +ve | Total (n=60, 100%) | p-value |
|---|
| (n=30, 50%) | (n=30, 50%) |
|---|
| Peritoneal nodules |
| Absent | 29 (96.67%) | 23 (76.67%) | 52 (86.67%) | 0.023 |
| Present | 1 (3.33%) | 7 (23.33%) | 8 (13.33%) | |
| EMVI |
| Absent | 29 (96.67%) | 29 (96.67%) | 58 (96.67%) | 1 |
| Present | 1 (3.33%) | 1 (3.33%) | 2 (3.33%) | |
| LVI |
| Absent | 23 (76.67%) | 10 (33.33%) | 33 (55%) | 0.0007 |
| Persent | 7 (23.33%) | 20 (66.67%) | 27 (45%) | |
| Perineural invasion |
| Absent | 23 (76.67%) | 13 (43.33%) | 36 (60%) | 0.008 |
| Persent | 7 (23.33%) | 17 (56.67%) | 24 (40%) | |
| LN metastasis |
| Absent | 19 (63.33%) | 5 (16.67%) | 24 (40%) | 0.0002 |
| Present | 11 (36.67%) | 25 (83.33%) | 36 (60%) | |
| Distant metastasis |
| Absent | 30 (100%) | 26 (86.67%) | 56 (93.33%) | 0.038 |
| Present | 0 (0%) | 4 (13.33%) | 4 (6.67%) | |
| TNM stage |
| Stage II | 17 (56.67%) | 7 (23.33%) | 24 (40%) | |
| Stage III | 13 (43.33%) | 19 (63.33%) | 32 (53.33%) | <0.01 |
| Stage IV | 0 (0%) | 4 (13.33%) | 4 (6.67%) | |
Lymph node metastasis, LVI, distant metastasis and tumour stage showed higher rates in pT3 cases with PELI than that of pT4a cases [Table/Fig-12].
The clinicopathological variables of positive PELI in pT3 CRC cases and pT4a CRC cases.
| Clinicopathologic features | PELI+ve pT3 CRC cases | pT4a CRC cases |
|---|
| (n=16, 26.7%) | (n=20, 33.3%) |
|---|
| Peritoneal nodules |
| Absent | 13 (81.25%) | 18 (90%) |
| Present | 3 (18.75%) | 2 (10%) p=0.78 |
| EMVI |
| Absent | 15 (93.75%) | 20 (100%) |
| Present | 1 (6.25%) | 0 (0%) p=0.909 |
| LVI |
| Absent | 5 (31.25%) | 14 (70%) |
| Present | 11 (68.75%) | 6 (30%) p=0.04 |
| Perineural invasion |
| Absent | 7 (43.75%) | 14 (70%) |
| Present | 9 (56.25%) | 6 (30%) p=0.21 |
| LN metastasis |
| Absent | 0 (0%) | 9 (45%) |
| Present | 16 (100%) | 11 (55%) p=0.006 |
| Distant metastasis |
| Absent | 12 (75%) | 20 (100%) |
| Present | 4 (25%) | 0 (0%) p=0.06 |
| TNM stage |
| Stage II | 1 (6.25%) | 9 (45%) |
| Stage III | 11 (68.75%) | 11 (55%) |
| Stage IV | 4 (25%) | 0 (0%) p=0.003 |
| D2-40 expression of lymphatic vessels |
| Negative | 1 (6.25%) | 10 (50%) |
| Positive | 15 (93.75%) | 10 (50%) p=0.009 |
Discussion
Among pT3 CRC cases, the wide range in the depth of tumour invasion may explain the variations in their clinicopathologic features and their clinical outcomes [14-16]. The tumour invasion of the visceral peritoneum may alter the microenvironment of the tumour and affect its progression [17-19]. Thus, classifying pT3 tumours into those without peritoneal membrane invasion (i.e., limited to the subserosa) and those with peritoneal membrane invasion separates these tumours into different prognostic groups [20,21].
Since, Shinto E et al., first introduced the importance of PELI to stratify pT3 CRC, many studies thereafter evaluated PELI [22]. However, PEL detection in pT3 CRC by elastic stain varies considerably among different studies. While Nakanishi Y et al., identified PEL in their 139 cases, Liang W et al., could not identify PEL in more than half of their cases (58.6%) [3,23]. In this work, PEL was unidentified in only 6.67%. Likewise, the proportion of pT3 with or without tumour invasion differs between studies. In current work, PELI was detected in 40% of the pT3 CRC cases. Though only one elastic stained-section was performed per case, this ratio was close to those reported by Kojima M et al., Shinto E et al., and Yokota M et al., who examined differing number of sections per tumour stained for elastic lamina where PELI was identified in 41.5% 52.6%, and 41.7% of the pT3 CRC cases respectively [17,22,24]. Meanwhile, Nakanishi Y et al., Liang W et al., and Grin A et al., performed only one elastic stain per case found PELI in lower rates 23.02%, 26.8% and 16.7% of the pT3 CRC cases, respectively [3,23,25]. This may be due to the careful selection of sections that demonstrated the closest area of tumour invasion to the surface of the peritumoural adipose tissue.
In this study, PELI was associated with adverse prognostic parameters. All pT3 positive PELI tumours showed lymph node metastasis (100%) while cases with negative PELI showed much lower lymph node metastasis (35%) in pT3 CRC cases (p-value=0.001). This was in agreement with studies of Nakanishi Y et al., and Shinto E et al., [3,22]. Similarly, a statistically significant relationship between the LVI (weather detected by H&E or using D2-40 immunostaining) and PELI status was detected in T3 CRC cases (p-value=0.009 and <0.001 respectively). This was consistent with the results of Nakanishi Y et al., and Kojima M et al., where LVI was detected in higher percentages in cases with PELI than cases without [3,17]. In contrast, Liang W et al., reported lack of differences between the PELI with respect to lymphovascular invasion [23]. PELI was associated with perineural invasion (statistically insignificant). This correlation was also reported by Laing W et al., but was of statistical significance [23].
In this work, all cases with distant metastasis showed PELI (statistically significant). It was consistent with the results of Shinto E et al., [22]. In addition, the majority of TNM stage III tumours (52.38%) and all cases of TNM stage IV tumours showed positive PELI (statistically significant, p-value <0.001), and this was in agreement with what was stated by Kojima M et al., [17]. Subsequently, pT3 CRC could be divided into 2 separate categories: with and without PEL invasion. Liang W et al., recommend future upstaging of pT3 tumours with PELI especially in those without lymph node metastasis [26].
In this work, D2-40 immunostaining objectively highlight LVI by tumour cells. D2-40 detected LVI was significantly correlated with H&E-LVI (p-value=0.0007). However, ten cases (16.7%) with D2-40-LVI invasion were not detected on H&E. Also, Saad R et al., and Walgenbach-Bruenagel G et al., reported that D2-40 increased the detection rate of angiolymphatic invasion by 19% and 46.3% respectively over routine H&E and stated the significant correlation between H&E and D2-40 detected LVI [27,28]. Meanwhile, walls of H&E-LVI lesions in 7 (11.7%) were not stained with D2-40, maybe because small venous vascular invasion could be misinterpreted as lymphatic invasion in H&E sections.
When D2-40 detected LVI is considered a predictive factor of lymph node metastasis in this study, sensitivity, specificity, positive predictive value, and negative predictive value are 69.44%, 79.17%, 83.33%, and 63.33%, respectively, the high incidence of positive predictive value indicates that D2-40-LVI is a useful indicator of nodal metastasis of colorectal cancer. These rates were slightly different from results of Ishii M et al., and Wada H et al., where the sensitivity, specificity, positive predictive value, and negative predictive value were 72.2%, 72.9%, 28.9% & 94.5% and 58%, 88%, 35% & 95%, respectively [29,30]. Wada H et al., explained the relative low positive predictive value of D2-40 LVI by the possibility that cancer cells invading lymphatics do not always colonise in a regional lymph node [30].
In addition to its predictive value to lymph node metastasis, D2-40 detected LVI was correlated with negative prognostic factors in CRC. It was significantly correlated with perineural invasion, presence of peritoneal nodules and distant metastasis as well as higher tumour stage. In concordance with the present results, Saad R et al., reported significant correlation between D2-40 detected LVI with the presence of liver metastasis (p-value <0.001) [27]. In addition, Walgenbach-Bruenagel G et al., notified that D2-40 identified LVI increased from 0% to 22%, 84% & 83% in stage I, II, III, and IV, respectively compared to 29.17%, 59.38% and 100% of stage II, III and IV CRC cases in the current work (statistically significant) [28].
When comparing PELI-positive pT3 with pT4a CRC, it seems that they have similar relations with some adverse prognostic markers. There was no statistical significant difference between them in respect to perineural invasion, distant metastasis, EMVI, and peritoneal nodules. Surprisingly, though pT3 was significantly associated with other adverse factors like LVI (detected by H&E or by D2-40 immunostaining), lymph node metastasis, higher stage when compared with pT4 CRC. More than half of studied pT4a CRC cases presented with lymph node metastatic deposits, while all CRC cases with positive PELI presented with lymph node metastatic deposits. LVI (H&E and D2-40) was detected in lower rate in studied pT4a CRC cases (30% and 50%) compared to pT3 cases with positive PELI (68.75% and 93.75%). In addition, the majority of pT4a CRC cases (55%) and 68.75% of CRC cases with positive PELI were stage III. One fourth of studied CRC cases with positive PELI were stage IV while none of pT4a CRC cases were stage IV. Similarly, Nakanishi Y et al., reported higher percentage of lymph nodes metastatic deposits in their CRC cases with positive PELI (87.5%) in comparison to 70% of their pT4a CRC cases were associated with lymph node metastasis. Furthermore, they reported lower LVI rates in studied pT4a CRC cases, 36.7% in relation to CRC cases with positive PELI 43.8% [3]. Accordingly, they recommend that pT3 CRC cases with PELI to be treated as pT4a cases and the results of this work support their recommendations.
Limitation
The limitation of this study was the small number of the collected sample.
Conclusion
PELI enhanced by elastic stain and D2-40 detected LVI could segregate a subgroup of T3 CRC that is associated with adverse prognostic factors, which would signify upstaging of pT3 with PELI into T4a CRC with resultant implication on patients’ treatment strategy.
Future Recommendations
Further studies on larger sample and follow-up are necessary to compare the outcomes of these currently distinct groups of patients.