Introduction
Polycystic ovary syndrome (PCOS) is associated with metabolic derangements including insulin resistance which causes dyslipidemia and glucose intolerance. In addition, low-grade inflammation reflected by elevated serum High-sensitivity C-Reactive Protein (hs-CRP) levels is common in PCOS patients. Non-pharmacological interventions such as exercise training can play an important role in reducing insulin resistance and inflammatory markers.
Aim
To determine the effect of 12-weeks of High Intensity Interval Training (HIIT) program on insulin resistance and hs-CRP in women with PCOS.
Materials and Methods
In this Quasi-experimental study, 24 female patients with PCOS were included and divided into two groups {HIIT (12 cases) and control group (12 cases)}. HIIT consisted of three sessions for 12-weeks of intense anaerobic exercise with short periods (4 minutes) to reach 90% of maximal heart rate for 2 sessions and 1 session with moderate exercise to reach 70% of maximal heart rate. The control group resumed normal daily life without any intervention. Serum hs-CRP and fasting glucose and insulin levels were measured at baseline and after 12-weeks and HOMA-IR was calculated. Paired t-test was used to compare the measured continuous variables.
Results
Mean (±SD) HOMA-IR in HIIT group decreased from 3.22 (±1.72) to 1.99 (±0.66); p=0.001. Mean (±SD) serum hs-CRP level in HIIT group decreased from 6.3 (±1.41) to 4.7 (±1.25) mg/L; p=0.033. No significant decrease was seen in control group.
Conclusion
HIIT is associated with improvement of insulin resistance and decrease in hs-CRP in PCOS patients. This exercise training can be used as early intervention in PCOS patients.
Introduction
Polycystic ovary syndrome (PCOS) is one of the common endocrinopathies in women most commonly diagnosed among women in reproductive age group (i.e., 20 to 40 years of age) with a prevalence of 5 to 6% [1]. PCOS is a form of hyperandrogenism in the ovaries. PCOS morphologic manifestations include ovarian enlargement of more than 9 mm, presence of 10 or more 2 to 8-mm cysts with clinical signs such as oligomenorrhea, anovulation, hirsutism and ovulation absence [2]. PCOS can cause metabolic abnormalities such as insulin resistance, obesity and dyslipidemia, as well as increased risk of cardiovascular disease and Type 2 Diabetes Mellitus (T2DM) [3,4].
The mechanism of insulin resistance in PCOS is not fully understood, but chronic mild inflammation has been suggested as one of the factors involved, similar to the one seen in obese patients where low-grade chronic inflammation can lead to insulin resistance by inflammatory markers such as Tumour Necrosis Factor (TNF)-α [5]. Mild chronic inflammation is associated with a higher than normal level of several cytokines, including TNF-α, Interleukin-6 (IL-6), and CRP [6,7]. Although insulin resistance is related to obesity and visceral fat, this state can be seen in PCOS patients who are not obese. However, metformin is not recommended routinely to treat insulin resistance in PCOS patients [8].
C-reactive protein (CRP) is a protein and acute phase reactant produced by the liver in response to inflammatory conditions such as infection or injury. Increased CRP level along with several other inflammatory markers has been demonstrated in PCOS. Increased CRP level has been documented in PCOS even after adjustments for age and Body Mass Index (BMI) [7].
Despite the benefits of exercise for the general public, there is still need for more studies regarding the effect of exercise in PCOS patients. Exercise training and its effects among PCOS patients have been done in some domains including body composition and reproductive function [9], insulin resistance and body composition [10], cardiorespiratory capacity [11], and so on. In a systematic review performed regarding exercise interventions in PCOS, it was concluded that due to high heterogeneity in outcomes and designs, definite conclusive results cannot be made [12].
Two main types of exercise namely aerobic exercise and resistance exercise or a combination of these two methods have been studied in PCOS patients. HIIT is generally referred to as repeated exercise sessions, which is relatively short in duration to reach maximal effort with some intervening rest time. HIIT is advocated as effective and time-efficient method to promote health [13,14]. In previous studies, the effect of HIIT in PCOS patients has been investigated. For instance, combined aerobic-resistance exercise with calorie restriction improved body composition of PCOS patients. However, this intervention did not affect reproductive function [15]. Another study demonstrated that HIIT for 10 weeks improved insulin resistance and hs-CRP in PCOS patients, but strength training did not affect hs-CRP level [16].
Although several studies have tried to demonstrate the efficacy of resistance exercise training on PCOS patients, there is need for further studies in this area. New studies with different designs and characteristics of the patient population could enhance the knowledge about the feasibility and efficacy of exercise training programs in this population. Therefore, the aim of this study was to determine the effect of 12-weeks of HIIT on insulin resistance and hs-CRP level in PCOS patients.
Materials and Methods
In this quasi-experimental study, 24 women diagnosed by PCOS were recruited. The study was conducted at a university hospital which is a tertiary referral centre with services for gynaecological disorders affiliated to Kermanshah University of Medical Sciences, Kermanshah, Iran. The study started from March 2017 and ended in September 2017.
During the study period, 24 women diagnosed by PCOS who agreed to participate in the study and provided blood samples were consecutively included. They were divided into HIIT group (12 cases) and control group (12 cases) who did not receive any intervention. The control group was instructed to resume routine daily lifestyle and diet.
Inclusion criteria were PCOS patients diagnosed or with a history of symptoms of PCOS for more than six months within age range of 20 to 40 years and not being involved in any other exercise training (during the preceding two months before study initiation) or physiotherapy/rehabilitation courses and without other chronic conditions such as infectious, immune disorders, musculoskeletal conditions, cardiovascular/neurologic/psychiatric diseases and visual disturbances. The diagnosis of PCOS was made according to the Rotterdam criteria [17]. According to the criteria, three factors are assessed by clinical, imaging, and biochemistry (oligo- and/or anovulation, clinical and/or biochemical signs of excess androgen, polycystic ovaries). If at least two of these criteria are present, the diagnosis of PCOS is confirmed.
Exclusion criteria were Cushing syndrome and androgen-secreting tumours, using walking aid devices, endocrine disorders such as hyperprolactinemia and hyperthyroidism, use of oral contraceptive, anti-diabetic, anti-androgenic treatments and oocyte induction or any corticosteroid substance within the past 30 days, pregnancy, smoking and alcohol consumption.
First, the goals of the study and the stages of the research were described for the subjects. After obtaining a letter of informed consent from the subjects, they were included. The study proposal and details were verified by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran with the ethics code IR.KUMS.REC.1397.573.
High Intensity Interval Training
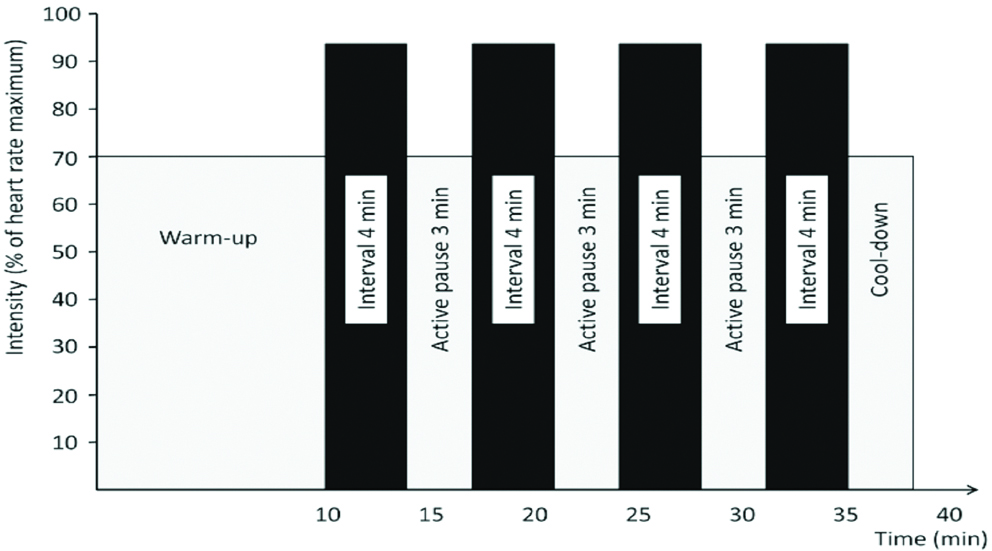
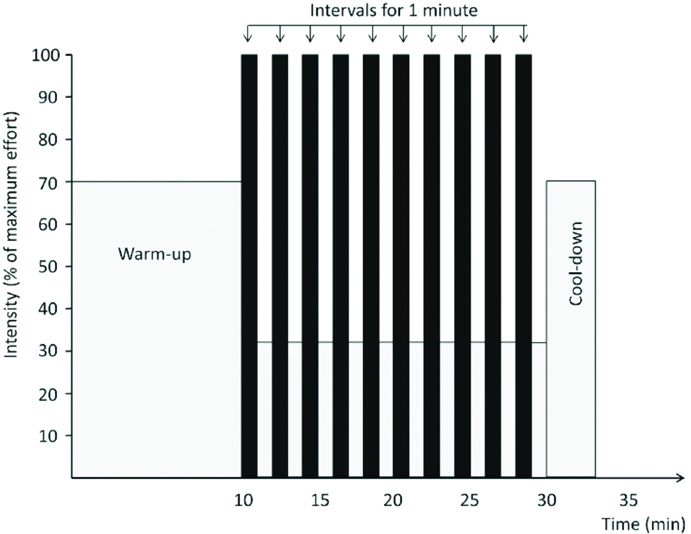
HIIT is a type of interval training (in comparison to continuous training) where subjects perform intermittent short bursts of high-intense anaerobic exercises with recovery periods between these short periods. During the recovery periods, the subjects perform less intense activity or they may rest [18]. In this study, HIIT was scheduled for 12-weeks (3 sessions per week). Of three weekly sessions, two sessions were planned with four stages of four-minute running with the goal of achieving 90 to 95% of maximum heart rate (reflecting high-intensity training). These four high-intense stages were separated by three stages of less-intense (moderate intensity) running with the goal of achieving a heart rate equal to 70% of maximum heart rate [Table/Fig-1]. One weekly session was scheduled with 10 periods of one-minute high-intense running separated by 10 one-minute periods of restring. The subjects performed the exercises in an indoor sports gym.
High intensity interval training with four stages of four-minute training with three moderate intensity stages between them.
The third session consisted of 10 stages of one-minute exercise with maximum intensity separated by a very low activity or one-minute rest stages between the high intensity stages [Table/Fig-2].
High intensity interval training with 10 stages of one-minute training with intervals of moderate exercise or rest between them.
The patients of HIIT group were instructed about the method of doing the exercises. In order to monitor and ensure that the HIIT program, each week the patients were randomly selected and contacted in presence to monitor the exercise training sessions.
Laboratory Assays
First, before the beginning of the study, hs-CRP, fasting insulin and fasting glucose levels were measured. These measurements were repeated 48-hours after completion of the study. About 3 mL of venous blood samples were taken from the anterior cubital vein. Sampling was performed at a specific time of day (8 to 10 AM), so that the level of insulin and CRP is not affected by its overnight fluctuations. Blood samples were centrifuged for 15 minutes at a rate of 3000 rpm and stored at -80°C. Measurement of fasting insulin level was done by sandwich ELISA using the isotope kit. Immunoturbidimetry was used to measure hs-CRP.
Homeostatic Model of Assessment of Insulin Resistance (HOMA-IR) was used to determine insulin resistance [19].
HOMA-IR = [fasting plasma glucose (mmol/L) × fasting insulin concentration (μU/mL)]/22.5Body Mass Index
The participants were weighted using Seca Digital Weigh with 0.1-kg precision. The subject’s height was measured by using measurement tape fixed on the wall with minimum 0.1 centimetres. The BMI was calculated as body weight in kg divided by height in meters squared.
Statistical Analysis
Descriptive indices (mean, standard deviation, frequency, and percentage) were used to describe the variables. Paired t-test was used to compare HOMA-IR and hs-CRP levels before and after HIIT. Student’s t-test was used to compare continuous variables between the two study groups. Statistical analyses were performed by SPSS software (version. 22.0) and significance level was considered as 5%.
Results
A total of 24 female patients were studied. There was no statistically significant difference regarding baseline variables between the two groups [Table/Fig-3].
Demographic characteristics of polycystic ovary syndrome patients in high intensity interval training (HIIT) and control groups.
| Variables | HIIT group (N=12) | Control group (N=12) | p-value |
|---|
| Age (years) | 34.34 (±4.69) | 32.18 (±3.47) | 0.34 |
| Height (cm) | 176.71 (±5.39) | 173.48 (±6.27) | 0.62 |
| Weight (kg) | 67.97 (±6.41) | 63.24 (±8.65) | 0.24 |
| BMI (kg/m2) | 21.19 (±1.74) | 21.33 (±2.62) | 0.41 |
Data are presented as mean (±standard deviation); BMI: Body mass index
There was a significant decrease in hs-CRP (25.4% decrease from baseline) and HOMA-IR (38.1% decrease from baseline) in the experimental group after HITT. However, no significant change was observed in control group [Table/Fig-4].
Comparison of hs-CRP and HOMA-IR before and after the study in high intensity interval training (HIIT) and control groups.
| Groups | Variables | Baseline | After 12-weeks | p-value |
|---|
| Experimental | Fasting glucose, mmol/L | 5.06 (±0.97) | 4.26 (±1.13) | <0.001* |
| Fasting insulin, μIU/mL | 13.98 (±8.14) | 9.92 (±8.76) | 0.006* |
| HOMA-IR | 3.22 (±1.72) | 1.99 (±0.66) | 0.001* |
| hs-CRP, mg/L | 6.3 (±1.41) | 4.7 (±1.25) | 0.033* |
| Control | Fasting glucose, mmol/L | 4.98 (±0.89) | 5.12 (±1.03) | 0.13 |
| Fasting insulin, μIU/mL | 13.56 (±8.52) | 14.02 (±9.12) | 0.23 |
| HOMA-IR | 2.86 (±1.17) | 2.71 (±0.96) | 0.95 |
| hs-CRP, mg/L | 6.6 (±1.57) | 6.8 (±1.61) | 0.82 |
Data are presented as mean (±standard deviation); *statistically significant
HOMA-IR: Homeostatic model of assessment of insulin resistance; hs-CRP: High-sensitivity C-reactive protein
Discussion
Chronic mild inflammation and metabolic abnormalities such as insulin resistance are associated with PCOS. Efforts have been made to investigate different interventions to improve insulin sensitivity and reduce inflammation. One such intervention is exercise training in the form of aerobic exercise or resistance training. The aim of this study was to determine the effect of 12-weeks of HIIT on the levels of insulin resistance and hs-CRP in women with PCOS. The results of the study showed that after 12-weeks of HIIT, both insulin sensitivity (a decrease in HOMA-IR) and hs-CRP levels improved significantly in this group. However, no meaningful change was seen in control group that did not receive any intervention. The results of this study are consistent with the results of some previous studies. In a previous study, including two methods of exercise training (strength training and HIIT) on eight PCOS patients, mean hs-CRP decreased from a baseline value of 3.2 mg/L to 2.4 mg/L which was not statistically significant [16]. In the mentioned study [16], other inflammatory markers (adiponectin and leptin) similar to hs-CRP did not change significantly. However, in agreement with the present findings, HOMA-IR improved significantly in HIIT group and deceased from a baseline mean value of 4.9 to 4.1. As stated previously, inflammation has been suggested as a mechanism for insulin resistance in PCOS patients. This was the reason that it was decided to measure HOMA-IR index as well as hs-CRP to find out whether any changes can occur in both of them. However, considering the results of the mentioned study [16] that HOMA-IR decreased and hs-CRP did not change, it may be concluded that other factors contribute to insulin resistance and inflammation is not the sole mechanism underlying inflammation in PCOS.
In another study by Thompson RL et al., comparing three interventions (diet, diet and exercise, and combined aerobic-resistance exercise) in PCOS patients, mean HOMA value at baseline was 2.04 which decreased to 1.41 after 20 weeks of combined aerobic-resistance training which was significant [15]. This finding is compatible with the current findings, although the methods of exercise training programs are different. In this study, only the effect of resistance training was studied. The women did not perform aerobic exercise. As mentioned above different exercise methods and settings is a major factor when comparing the findings between different studies. Hutchison et al., [10] investigated 12-weeks of intensified exercise intervention (three hours weekly) on two groups of women with (13 subjects) and without (eight subjects) PCOS. All subjects had BMI of more than 27 kg/m2. In PCOS group, HOMA had a median of 5 [interquartile range=3.7 to 8.2] at baseline. Although this decreased to 3.4 [2.3 to 9.2], the change was not statistically significant (p=0.43). This finding is not compatible with the present results. The difference could be attributed to several factors. The subjects in the present study mostly had normal BMI with a mean value of 21 kg/m2 in HIIT group. However, all patients in the mentioned study [10] had BMI of >27 with an average of 35 kg/m2 which significantly decreased after 12-weeks of exercise. Despite this decrease in BMI value, visceral fat did not change. This highlights the role that visceral fat plays regarding insulin resistance. However, the authors [10] did not show a causational relationship between visceral fat and insulin resistance. As study designs and frequency and intensity of exercise programs differ among studies, there is still a need for further well-controlled studies to reach definite conclusions. Another issue that should be considered in PCOS patients is that insulin resistance may not be necessarily present in all PCOS patients irrespective of their BMI. Besides, those with insulin resistance may have varying severities of insulin resistance. Some studies have determined combined effect of diet and energy restriction in addition to physical exercise in PCOS women [20]. As here the present authors did not control the diet of the subjects and only physical exercise was selected as the intervention, head-to-head comparisons cannot be made with studies that controlled both diet and physical exercise. In addition, not all PCOS women have obesity and BMI values greater than 25 kg/m2. Since many patients with PCOS may have BMI values in the range of obesity, many former studies have recruited PCOS patients with obesity [10,16].
An important mechanism proposed for the role of exercise in insulin resistance is the glucose uptake by skeletal muscle which occurs via activation of signalling pathways [21]. Exercise can stimulate molecular signalling pathways and by facilitating GLUT4 expression, ultimately leads to glucose transport into the cell [22]. This mechanism is extensively studied in diabetic patients. The signalling pathways are dependent on insulin action and the skeletal muscle is the main site of this action. Besides, insulin resistance is well established to have correlation with visceral fat [23]. The change in the weight of the patients was not evaluated to determine whether abdominal fat had correlation with the observed findings. Insulin resistance is a major biochemical concern in obese patients as well as in PCOS patients. Insulin resistance can have consequences regarding cardio-metabolic outcomes. Therefore, it should be managed properly in PCOS patients to prevent development of future metabolic abnormalities such as T2DM. As mentioned earlier, there is controversy regarding initiating metformin routinely in PCOS patients. Therefore, non-pharmacologic therapies such as HIIT not only can have beneficial effect on insulin resistance but can have other benefits for overall health.
It is recommended that in the future studies, clinical trials with larger sample size with randomisation and longer follow-up be performed to overcome the limitations encountered in this study. In addition, controlling the diet of the patients, will provide more robust information about the role of exercise training. In addition having different exercise training programs will enable us to compare the feasibility and efficacy of different methods of exercise to find out which training has the best outcome.
Limitation
There were certain limitations in this study. First, except for hs-CRP, other inflammatory markers were not measured. Also, body composition and weight changes were not investigated. In addition, more patients were not accessible for inclusion in the study and randomisation was not implemented here. However, as BMI was comparable between the two groups at baseline, it could be concluded that weight and body position may not have significant effect on the findings. Also, the diet of the patients was not controlled in either group and calorie counting was not performed.
Conclusion
HIIT for 12-weeks had significant effect on insulin sensitivity and hs-CRP in PCOS patients. These findings confirm the beneficial role of regular resistance exercises in improving insulin sensitivity. It is suggested that PCOS women perform resistance exercises.
Data are presented as mean (±standard deviation); BMI: Body mass index
Data are presented as mean (±standard deviation); *statistically significant
HOMA-IR: Homeostatic model of assessment of insulin resistance; hs-CRP: High-sensitivity C-reactive protein
[1]. Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G, The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysisOncotarget 2017 8(56):96351-58.10.18632/oncotarget.1918029221211 [Google Scholar] [CrossRef] [PubMed]
[2]. Sirmans SM, Pate KA, Epidemiology, diagnosis, and management of polycystic ovary syndromeClin Epidemiol 2013 6:01-13.10.2147/CLEP.S3755924379699 [Google Scholar] [CrossRef] [PubMed]
[3]. Pirwany IR, Fleming R, Greer I A, Packard CJ, Sattar N, Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parametersClin Endocrinol (Oxf) 2001 54(4):447-53.10.1046/j.1365-2265.2001.01228.x11318779 [Google Scholar] [CrossRef] [PubMed]
[4]. Carmina E, Lobo RA, Use of fasting blood to assess the prevalence of insulin resistance in women with polycystic ovary syndromeFertil Steril 2004 82(3):661-65.10.1016/j.fertnstert.2004.01.04115374711 [Google Scholar] [CrossRef] [PubMed]
[5]. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y, Obesity and inflammation: the linking mechanism and the complicationsArch Med Sci 2017 13(4):851-63.10.5114/aoms.2016.5892828721154 [Google Scholar] [CrossRef] [PubMed]
[6]. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S, Interleukin-6 Levels in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-AnalysisPLoS One 2016 11(2):e014853110.1371/journal.pone.014853126849353 [Google Scholar] [CrossRef] [PubMed]
[7]. Duleba AJ, Dokras A, Is PCOS an inflammatory process?Fertil Steril 2012 97(1):7-12.10.1016/j.fertnstert.2011.11.02322192135 [Google Scholar] [CrossRef] [PubMed]
[8]. Marshall JC, Dunaif A, Should all women with PCOS be treated for insulin resistance?Fertil Steril 2012 97(1):18-22.10.1016/j.fertnstert.2011.11.03622192137 [Google Scholar] [CrossRef] [PubMed]
[9]. Harrison CL, Stepto NK, Hutchison SK, Teede HJ, The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndromeClin Endocrinol (Oxf) 2012 76(3):351-57.10.1111/j.1365-2265.2011.04160.x21711376 [Google Scholar] [CrossRef] [PubMed]
[10]. Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ, Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndromeJ Clin Endocrinol Metab 2011 96(1):E48-56.10.1210/jc.2010-082820926534 [Google Scholar] [CrossRef] [PubMed]
[11]. Delgado-Floody P, Latorre-Roman P, Jerez-Mayorga D, Caamano-Navarrete F, Garcia-Pinillos F, Feasibility of incorporating high-intensity interval training into physical education programs to improve body composition and cardiorespiratory capacity of overweight and obese children: A systematic reviewJ Exerc Sci Fit 2019 17(2):35-40.10.1016/j.jesf.2018.11.00330740131 [Google Scholar] [CrossRef] [PubMed]
[12]. Harrison CL, Lombard CB, Moran LJ, Teede HJ, Exercise therapy in polycystic ovary syndrome: a systematic reviewHum Reprod Update 2011 17(2):171-83.10.1093/humupd/dmq04520833639 [Google Scholar] [CrossRef] [PubMed]
[13]. Gibala MJ, High-intensity interval training: a time-efficient strategy for health promotion?Curr Sports Med Rep 2007 6(4):211-13.10.1097/01.CSMR.0000306472.95337.e917617995 [Google Scholar] [CrossRef] [PubMed]
[14]. Eddolls WTB, McNarry MA, Stratton G, Winn CON, Mackintosh KA, high-intensity interval training interventions in children and adolescents: a systematic reviewSports Med 2017 47(11):2363-74.10.1007/s40279-017-0753-828643209 [Google Scholar] [CrossRef] [PubMed]
[15]. Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD, The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndromeJ Clin Endocrinol Metab 2008 93(9):3373-80.10.1210/jc.2008-075118583464 [Google Scholar] [CrossRef] [PubMed]
[16]. Almenning I, Rieber-Mohn A, Lundgren KM, Løvvik TS, Garnæs KK, Moholdt T, Effects of high intensity interval training and strength training on metabolic, cardiovascular and hormonal outcomes in women with polycystic ovary syndrome: a pilot studyPLoS One 2015 10(9):e013879310.1371/journal.pone.013879326406234 [Google Scholar] [CrossRef] [PubMed]
[17]. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS)Hum Reprod 2004 19(1):41-47.10.1093/humrep/deh09814688154 [Google Scholar] [CrossRef] [PubMed]
[18]. Norton K, Norton L, Sadgrove D, Position statement on physical activity and exercise intensity terminologyJ Sci Med Sport 2010 13(5):496-502.10.1016/j.jsams.2009.09.00820005170 [Google Scholar] [CrossRef] [PubMed]
[19]. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia 1985 28(7):412-9.10.1007/BF002808833899825 [Google Scholar] [CrossRef] [PubMed]
[20]. Nybacka A, Carlstrom K, Stahle A, Nyren S, Hellstrom PM, Hirschberg AL, Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndromeFertil Steril 2011 96(6):1508-13.10.1016/j.fertnstert.2011.09.00621962963 [Google Scholar] [CrossRef] [PubMed]
[21]. Richter EA, Hargreaves M, Exercise, GLUT4, and skeletal muscle glucose uptakePhysiol Rev 2013 93(3):993-1017.10.1152/physrev.00038.201223899560 [Google Scholar] [CrossRef] [PubMed]
[22]. Rohling M, Herder C, Stemper T, Mussig K, Influence of Acute and Chronic Exercise on Glucose UptakeJ Diabetes Res 2016 2016:286865210.1155/2016/286865227069930 [Google Scholar] [CrossRef] [PubMed]
[23]. Patel P, Abate N, Body fat distribution and insulin resistanceNutrients 2013 5(6):2019-27.10.3390/nu506201923739143 [Google Scholar] [CrossRef] [PubMed]