Cell Block (CB) refers to the collecting of sediment, blood clots or grossly visible pieces of tissue from cytologic specimens that are processed into paraffin blocks and stained with H&E stain [1,2]. The CB preparation for microscopic evaluation was first introduced by Bahrenburg in 1896 [3-5]. In the words of Koss L “The CB technique should be used for processing all residual material remaining after completion of cytological preparations. This material often contains useful information” [1].
Various methods of CB preparation has been described since ancient times which include the one without any holding media which is the simplest of all (Fixed sediment method) [1], while the other methods require holding media like bacterial agar [1,2], plasma thromboplastin method [1,2,6], simplified cell block method [1,2], compact CB method [1,2], vapour fixation method [7] and histogel method to name a few [1,2,8]. Among these, the Plasma Thromboplastin method of Cell Block (PTCB) is simple, less time consuming, reproducible and economical as this can be done by using in-house raw materials like plasma and thromboplastin commonly found in blood bank and haematology laboratory [1,7,9].
Routine processing of CBs on an average, be it either with Histokinette varies between 12-24 hours [1] or manual processing would require atleast 5-6 hours (for small biopsies) [1,8]. This causes a delay in the additional information CBs can provide, one of the important reasons why CBs are attempted. Hence techniques like microwave processing are the need of the hour for faster processing especially in this era of neoadjuvant and targeted therapies [2,8]. Furthermore, we are now in the age of personalised medicine, where metastases are targeted for diagnosis mainly by Fine Needle Aspiration (FNA) [2,10].
Materials and Methods
This observational study was undertaken in the Cytopathology section, Department of Pathology, Kempegowda Institute of Medical Sciences, Bengaluru, Karnataka, India. Over a period of two months, all the aspirates, (eighty (80) from routine FNAs conducted in the cytopathology section were included. After obtaining informed consent, an exclusive needle pass was made for CB preparation in co-operative patients. In rest of the cases, material remaining after making routine FNA smears was used.
FNA material (separately collected for CB or remaining after making smears) was rinsed with phosphate buffer solution and subjected to centrifugation @1500 rpm for 5 minutes (REMI CENTRIFUGE). The supernatant was discarded and 3 drops of outdated plasma [Table/Fig-1a], (obtained from blood bank) was added to the sediment and mixed well. This was followed immediately by adding 3 drops of thromboplastin [Table/Fig-1b], quickly to the plasma-sediment mixture and waited for 5-10 minutes for the formation of clot [Table/Fig-1c], in the same plastic test tube. Later, the clot thus formed was slid into the Whatman filter paper (No.52, WR BALSTON LTD., 11 cm disc) [Table/Fig-1d], held securely, wrapped and placed in routine histokinette plastic tissue cassettes [Table/Fig-1e] and transferred to a Borosil beaker [Table/Fig-1f] for microwave processing. A modified Bellotti’s technique [11] was used for microwave processing. A domestic microwave oven with low (20°C-30°C), medium (40°C-55°C) and high (70°C-90°C) temperature settings [Table/Fig-1g] was used for processing the samples as follows:
The various stages of PTCB preparation and processing (refer text).
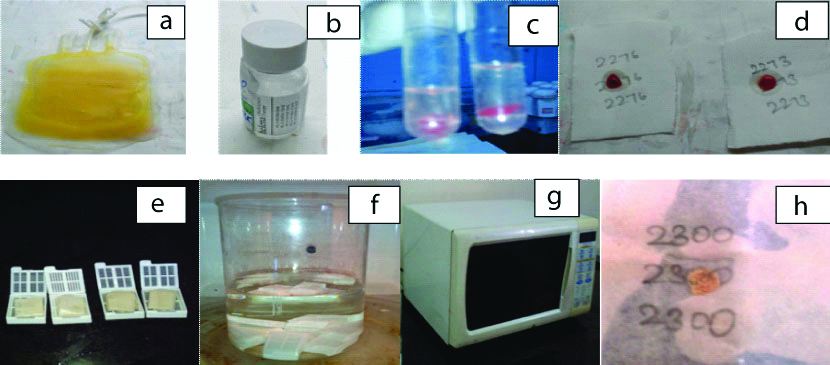
Fixation-10% buffered formalin for 1 minute @ medium heat (55°C) setting [11]
Dehydration with 50% ethyl alcohol for 1 minute @ low heat and 70% ethyl alcohol for one minutes @ low heat (30°C) setting [11]
Clearing with xylene for 1 minute @ low heat (30°C) →2 cycles [11]
Wax impregnation for 2½ minute @ high heat (70°C) →2 cycles [11].
The following precautions were taken while carrying out microwave processing:
A well ventilated room with exhaust fan.
Compulsory Personal Protective equipment which includes hand gloves, mask, goggles and cap.
Handling the reagents and glass beakers with utmost care to avoid inhalation of fumes.
Thus, the material from routine FNA’s from PT method of CB were ready for embedding after this short processing cycle of less than 10 minutes [Table/Fig-1h]. After this, tissues were embedded in paraffin wax. They were given a unique identification number, and the sections were cut at 4-5 microns and stained with Haematoxylin-Eosin (H&E) for microscopic examination. Special stains and IHC were attempted in required cases. Thus, both the FNA smears and PTCB processed through microwave processing were available on the same day and almost the same time for examination.
With all the available clinical data, the diagnosis on smears was compared to that with PTCB. The adequacy of material on PTCB was assessed and whether it contributed in reforming the diagnosis by performing additional ancillary tests like IHC in feasible cases. The microsections and final diagnosis on microwave processed PTCB was compared with respective histopathology sections wherever available.
Results
In this study, 80 cases from routine FNA’s were included. Among these, FNA’S from Breast lump constituted the majority of cases with 27.5% (22/80) [Table/Fig-2]. Females constituted 75% (60) and males 25% (20) [Table/Fig-3].
Distribution of cases (n=80).
| Site of FNA | Frequency (n) | Percentage (%) |
|---|
| Breast | 22 | 27.5% |
| Thyroid | 20 | 25% |
| Lymph node | 11 | 13.75% |
| Parotid glands | 08 | 10% |
| Lipoma | 08 | 10% |
| Lung mass | 01 | 1.25% |
| Axillary swelling | 02 | 2.5% |
| Epidermal cysts | 02 | 2.5% |
| Testicular swelling | 02 | 2.5% |
| Miscellaneous | 04 | 05% |
| Total | 80 | 100% |
| Site of FNA | Female (n) | Male (n) | Total (n) |
|---|
| Breast | 20 | 02 | 22 |
| Thyroid | 20 | 00 | 20 |
| Lymph node | 08 | 03 | 11 |
| Parotid glands | 03 | 05 | 08 |
| Lipoma | 05 | 03 | 08 |
| Lung | 00 | 01 | 01 |
| Axillary swelling | 02 | 00 | 02 |
| Epidermal cysts | 00 | 02 | 02 |
| Testicular swelling | 00 | 02 | 02 |
| Miscellaneous | 02 | 02 | 04 |
| Total | 60 (75%) | 20 (25%) | 80 |
Of the 80 cases, material was adequate in 56 of them i.e., in 70%, while in the remaining 24 cases material was not present in PTCBs. All the breast aspirates had adequate material in PTCBs while majority of the thyroid aspirates (65%) did not show contributable material. All (100%) aspirates from Lipomas did not show any material in PTCBs [Table/Fig-4].
FNA and material present on PTCB.
| Site | Material present (n) | Material not present (n) | Total (n) |
|---|
| Breast | 22 | 00 | 22 |
| Thyroid | 07 | 13 | 20 |
| Lymph node | 10 | 01 | 11 |
| Parotid swelling | 07 | 01 | 08 |
| Lipoma | 00 | 08 | 08 |
| Lung mass | 01 | 00 | 01 |
| Axillary swelling | 02 | 00 | 02 |
| Epidermal cysts | 02 | 00 | 02 |
| Testicular swelling | 02 | 00 | 02 |
| Miscellaneous | 03 | 01 | 04 |
| Total | 56 (70%) | 24 (30%) | 80 |
Of these 56 cases, 32 (58%) of them contributed in the diagnosis and helped in faster diagnosis [Table/Fig-5] whereas in the remaining 24 cases (42%), the contribution was not commendable. Cellular aspirates of lymph node, breast and tumourous tissue were processed well with PTCB and microwave processing. The quality of the sections, staining, morphology of cells and architecture were comparable to routine paraffin sections.
Distribution of cases contributing in the diagnosis.
| Sl. No. | Site | FNA | PTCB | HPE |
|---|
| 1 | Breast | Positive for malignancy | Intraductal carcinoma IHC | IDC-NOS |
| 2 | Breast | Positive for malignancy | Intraductal carcinoma IHC | IDC-NOS |
| 3 | Breast | Breast abscess | Breast abscess | --- |
| 4 | Breast | Fibroadenoma | Fibroadenoma | Fibroadenoma |
| 5 | Breast | Benign breast disease | Fibroadenoma | Proliferative breast disease without atypia |
| 6 | Breast | Subareolar abscess | Breast abscess | --- |
| 7 | Breast | Fibrocystic disease | Fibrocystic disease | Fibrocystic disease |
| 8 | Breast | Fibroadenoma | Fibroadenoma | --- |
| 9 | Breast | Lactational changes | Breast abscess | --- |
| 10 | Breast | Fibroadenoma | Fibroadenoma | Fibroadenoma |
| 11 | Breast | Fibrocystic disease | Fibrocystic disease | --- |
| 12 | Breast | Fibrocystic disease | Fibrocystic disease | --- |
| 13 | Parotid swelling | Acute suppurative process | Acute suppurative process | --- |
| 14 | Parotid swelling | Positive for malignancy | Mucoepidermoid carcinoma | Mucoepidermoid carcinoma |
| 15 | Parotid swelling | Positive for malignancy | Carcinoma ex pleomorphic adenoma | Carcinoma ex pleomorphic adenoma |
| 16 | Parotid swelling | Pleomorphic adenoma | Pleomorphic adenoma | Pleomorphic adenoma |
| 17 | Parotid swelling | Sialadenosis | Sialadenosis | --- |
| 18 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 19 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 20 | Lymph node swelling | Granulomatous lymphadenitis | Granulomatous lymphadenitis | Tuberculous lymphadenitis |
| 21 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 22 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 23 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 24 | Lymph node swelling | Reactive node | Reactive lymph node | --- |
| 25 | Axillary Lymph node swelling | Positive for malignancy | Intraductal carcinoma -breast | --- |
| 26 | Swelling axillary region | Suggestive of abscess | Acute suppurative inflammatory process | --- |
| 27 | CT guided FNA lung mass | Suspicious for malignancy | Suspicious of squamous cell carcinoma/poorly differentiated carcinoma | CT guided biopsy suggestive of squamous cell carcinoma |
| 28 | Swelling on forehead | Epidermal cyst | Epidermal cyst | Keratinous cyst |
| 29 | Swelling on neck | Epidermal cyst | Epidermal cyst | Keratinous cyst |
| 30 | Thyroid | Colloid goiter | Colloid goiter | --- |
| 31 | Thyoid | Colloid goiter with cystic change | Colloid goiter | --- |
| 32 | Thyroid | Multinodular goiter | Haemorrhage | --- |
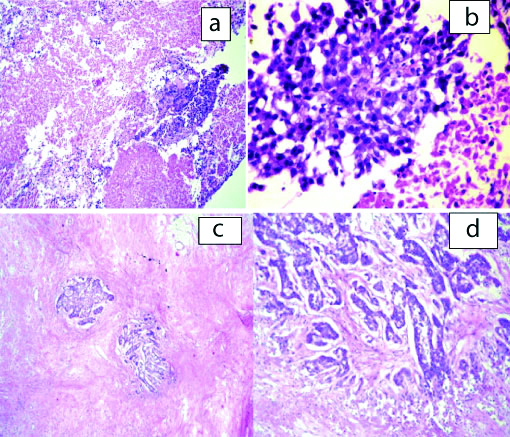
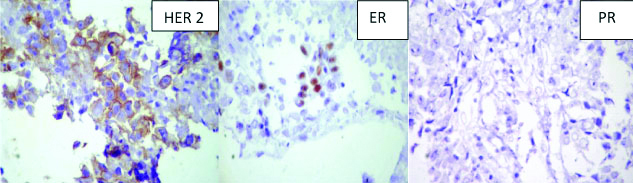
In 2 of the cases, with lump in the breast routine FNA suggested malignancy while on PTCB a definite diagnosis of infiltrating ductal carcinoma [Table/Fig-6] could be given which was confirmed by histopathology. IHC was attempted on the PTCB and was comparable with the IHC on routine HPE blocks [Table/Fig-7]. Two cases of swelling in the parotid region were given a diagnosis of smears positive for malignancy on routine FNA. However, PTCB upgraded the diagnosis to suspicious for mucoepidermoid carcinoma in one case and the other as carcinoma ex pleomorphic adenoma. Both these diagnoses were confirmed on histopathology [Table/Fig-8]. One case of axillary swelling in an elderly female patient was reported as positive for malignancy on FNA, whereas glandular and tubular arrangement of malignant cells was obtained on PTCB [Table/Fig-9]. One case of lower lobe lung mass was given suspicious for malignancy on CT guided FNA smears which were upgraded to suspicious of squamous cell carcinoma/poorly differentiated carcinoma on PTCB. On CT guided biopsy, the same case turned out to be squamous cell carcinoma [Table/Fig-5].
PTCB microsections from a case of IDC breast, H&E-10X (a) and 40X (b); corresponding histopathological microsections, H&E 10X (c) and 40X (d).
IHC on PTCB microsections in a case of IDC breast (IHC, DAKO).
PTCB microsections from a case of MEC (H&E-10X) corresponding histopathological microsection (H&E 40X).
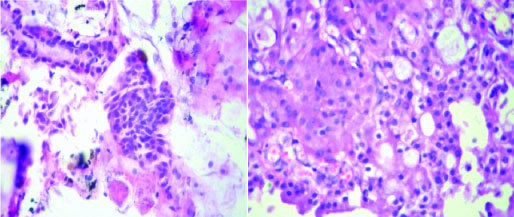
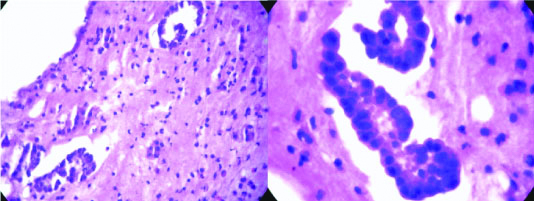
PTCB microsections from a case of metastatic IDC breast in an axillary lymph node, (H&E-10X) and corresponding histopathological microsection (H&E-40X).
Discussion
The conventional smear technique on body fluids surpassed the CB method after worldwide acceptance of Papanicolaou’s methods, though CBs were in use since the 1900s [1]. One of the constraints of conventional FNA smear is limited material available for adjuvant diagnostic investigations [1,12,13]. The CB technique has been applied to aspirated material [9] and has been used increasingly to improve the diagnostic accuracy of FNA.
Karnauchow PN et al., were the first to emphasise that CB technique could be used in thick tissue particles aspirated by FNA, which could provide sufficient material for a good section, special stains and IHC [9]. The CB technique helps in retrieval of small tissue fragments [1,3,4,8], improving cellular yield and diagnostic accuracy and provides scope for ancillary studies like IHC and special stains [1,2,4,5].
PTCBs for FNA and serous effusions by routine processing have been studied by Kulkarni MB et al., and Karnauchow PN et al., [6,9]. They concluded that PTCB method complements routine smears and thus aids in diagnosis with morphology comparable to routinely processed CB and an additional advantage of ability to do IHC.
We chose PTCB, as the method is simple and can be performed with minimal reagents which are readily available from the pathology laboratory.
Some of the disadvantages encountered were waiting time to bring the plasma to room temperature and difficulty in differentiating necrosis from proteinaceous material but routine processing of PTCB would require 18 to 24 hours/block.
The application of microwaves in histopathology has gained acceptance in last 2-3 decades [8]. It has been virtually used for all procedures in histopathology like stabilising tissues, fixation, processing, staining, frozen techniques, immune techniques, electron microscopy and antigen retrieval [8,14,15]. Rarely tried in cytology, microwave processing has been studied in preparing CB from sputum [16], direct FNA samples [11] and cervico vaginal smears [5].
As early as in 1986, Kok LP et al., used microwave technique for preparation of that CB from sputum which proved that CB could be prepared in approximately or even less than one hour [16]. A study by Bellotti MS et al., used a rapid method for processing of materials collected directly by FNA using microwave technology [11]. Microwave processing in cytology using routine CB in cervico-vaginal smears is done by Gangane N et al., [5].
Routine processing of PTCB would vary between 5-6 hours (manual processing) to 18-24 hours/block (automatic tissue processing using histokinette) while microwave processing decreases the turnaround time drastically to less than an hour [1,8,11].
In our study, the microwave processing of CBs on an average was 10-15 minutes/block (however inclusion of the time taken for PT method of CB preparation-1.5 to 2 hours) This is in concordance with the study by Prabhala S et al., who have combined PTCBs with microwave processing [17].
In our study, the quality of the sections, staining, morphology of cells and architecture are comparable to routine paraffin sections similar to other studies in addition to being both laboratory personnel and environment friendly [5,16-19]. The microwave processed CBs had better cytoplasmic and nuclear details with good erythrocyte integrity than the conventional method in concordance with Mathai AM et al., [18]. Kok LP et al., opined there is homogenous distribution of immersion fluids on the cells in microwave processing and hence the sections were comparably of superior quality [16]. CB technique provides archival material for future use. Also, various studies opine that antigenic epitopes are likely to be preserved better in microwave processed tissue. [5,8]. The material in CBs get concentrated in one small focal plane, which helps in faster and better evaluation [5] Overall, there was drastic reduction of time [8,13,16-18] and the microwave processed PTCB were available in few hours for comparison to the routine FNA smears. IHC was possible in microwave processed PTCBs in our study similar to other studies [5,8,12,14,15]. Skov BG et al., successfully tested Programmed Death ligand 1 IHC on PTCB method [20].
Cellular aspirates of lymph node, breast and tumourous tissue processed well with PTCB and microwave processing. In aspirates from thyroid, fibrous/neural and lipomatous lesions, PTCB did not yield material. Paucity of aspirate on routine FNA could be a factor in fibrous/neural tissue. In thyroid lesions, 24G needle is used, as it is a vascular organ and hence could lead to sparse cellularity. Also, needling is preferred over aspiration in anticipation of intra-organ haemorrhage leading to inadequate material for CBs. Majority of the thyroid cases which did not have material on PTCB were benign lesions like colloid goiter, some with cystic change on FNA smear examination. In lipomatous lesions, none of them showed material on PTCB possibily because adipocytes would have floated during centrifugation and pipetted off along with the supernatant.
In this era of molecular pathology and targeted therapy, many laboratories use CB’s for molecular techniques such as polymerase chain reaction-based sequencing, in situ hybridization and quantitative polymerase chain reaction to detect oncogene mutations, gene fusions, gene copy number gains, and protein overexpression. Despite the fact that, suboptimal cellularity is a common limitation of CBs, they are now feasible for next generation sequencing with the recommended cell content of minimum 500 tumour cells for various molecular techniques [2,21-24]. Various studies and research are being carried out for diagnosis and staging of metastases by FNA [2,10] and microwave processed PTCB could be a boon. In addition, CBs are of paramount importance for the bio-bank storage of cytological material in this bio banking millennium [2].
Limitation
Expertise and precision is required.
The process is labor intensive because the solutions are manually manipulated.
Conclusion
Microwave processing is possible on PTCBs and morphology of cells and architecture are comparable to that of routinely processed CB. IHC is possible in microwave processed PTCBs. Microwave processing decrease the turnaround time significantly and complemented smears in diagnosis. These could help in planning and strategising neoadjuvant therapy in cases of malignancy and hence decrease morbidity of extensive surgery.
Future Recommendation
Studies in large numbers are essential.