Ventilator Associated Pneumonia (VAP) complicates a significant proportion of mechanically ventilated patients in the Intensive Care Units (ICU) [1,2]. This nosocomial infection results in increased morbidity, mortality, duration of hospital stay and increased cost to the patient and the health care system [1]. Multiple non-pharmacological and pharmacological methods have been shown to be of benefit in the prevention of VAP [2,3]. However, the incidence of VAP remains high. Probiotics offer a novel approach to VAP prevention through mechanisms that include up-regulation of the host’s immune system and prevention of oral and gut colonisation by potentially pathogenic microorganisms and their subsequent micro-aspiration that is central to the pathogenesis of VAP [4]. Studies that have sought to ascertain the benefit of probiotics in the prevention of VAP have been difficult to compare because: heterogeneity in patient characteristic, strain of probiotic used, dose of probiotic used, method of administration and diagnostic criteria for VAP. Two recent meta-analysis concluded that the efficacy of probiotics for the prevention of VAP remains unclear [5,6] so, too its effect on mortality, morbidity, duration of ventilation or hospital stay. Also, there are no published Indian studies (in adult patients) which address this question.
This study was thus undertaken to ascertain the efficacy of administration of probiotic compound in the prevention of VAP in critically ill, mechanically ventilated patients in the medical ICU of a tertiary level hospital in Southern India.
Materials and Methods
This was a randomised, double-blinded, placebo controlled trial, to study the efficacy of the probiotic, Lactobacillusrhamnosus, as compared to placebo, in the prevention of VAP in mechanically ventilated patients in the MICU. The study was conducted at the Christian Medical College and Hospital, Vellore, Tamil Nadu, India. The institutional review board of this 2450 bed, tertiary, teaching hospital approved the study protocol prior to enrolling any patient. (Ethical approval letter number- 7617, dated 21.09.2011). All intubated patients admitted to the 24 bedded MICU and Medical High Dependency Unit (MHDU) was screened for eligibility for inclusion.
Exclusion criteria were aimed at excluding those patient groups, who were previously described as, or were at a theoretical risk for iatrogenic probiotic infection. Patients with cardiac valve or vascular grafts, rheumatic heart disease, previous rheumatic fever, congenital cardiac anomalies were excluded as they had a theoretical risk of developing a valvular endocarditis. Those with oropharyngeal, gastrointestinal or intestinal mucosal injury were excluded anticipating a possible increase in bacteraemia. Immuno-suppressed and pregnant patients were also similarly excluded.
Intubated adults over the age of 18 were screened and eligible for enrolment if there was a high likelihood that they would require intubation for more than 72 hours. Patients were enrolled between February 2012 and August 2012. Once the eligibility was established, patients were randomly assigned to one of the two study arms in a 1:1 ratio using computer generated randomisation codes. Block randomisation was used to generate the sequences with variable block sizes of 4, 6 and 8. Allocation concealment was achieved by sealing both the placebo and intervention capsules (identical in all aspects) (Unique Biotech, Hyderabad) in sequentially numbered containers according to allocation sequence. The researchers/outcome assessors, bedside clinicians and nursing staff were all blinded to group assignments.
Patients were enrolled after informed surrogate consent. Patients randomised to probiotic therapy received 2×109 CFU of Lactobacillus rhamnosus on a twice daily basis. The contents of one capsule containing 109 CFU of Lactobacillus were suspended in sterile water and given through a nasogastric tube. The contents of the second capsule were suspended in a sterile; water based surgical lubricant and applied as slurry to the oropharynx. The same method was used to deliver the contents of the identically appearing placebo capsule which contained only the base powder (i.e., without the Lactobacillus).
Patients continued to receive the active intervention or placebo for a total of seven days or until extubation, whichever was earlier. Patients also received all routine care including measures for the prevention of VAP as per the existing MICU protocols. There were no changes in these protocols throughout the study period.
On inclusion into the study, the baseline data collected included the medical history, demographic data and APACHE II score. Additional information collected on a daily basis included chest radiograph findings, clinical signs of VAP, length of stay in the ICU and in the hospital, duration of mechanical ventilation and any adverse events. The diagnosis of VAP was made based on the clinical criteria for VAP as described by Johanson WG et al., [7] (which required the presence of new and persistent infiltrates on the chest x-ray consistent with a diagnosis of VAP along with two out of three of the following- Fever/hypothermia, leukocytosis or leucopenia and purulent sputum).
Microbiological cultures were sent for patients diagnosed to have a VAP; the samples were obtained by tracheo-bronchial aspiration. A colony count of greater than 105 was considered significant and was correlated with the gram stain of the same. The Clinical Pulmonary Infection Score (CPIS) [8] was also calculated at baseline and after 72 hours of suspecting a VAP. The primary outcome was VAP diagnosed by clinical criteria. The secondary outcomes included duration of mechanical ventilation, ICU stay, hospital stay and mortality.
Blood cultures were collected as and when deemed clinically appropriate by the treating physician or intensivist. The co-morbid conditions were defined as follows: Diabetes-Self report of a previous diagnosis of diabetes or self-report of anti-diabetic medications use.
Hypertension-Self report of a previous diagnosis of hypertension or self-report of anti-hypertensive medication use.
Chronic obstructive pulmonary disease (COPD): self report of previous diagnosis of COPD or use of metered dose inhalers or previous spirometry consistent with a diagnosis of COPD.
Current smoker: Person who has smoked at least 100 cigarettes in his or her lifetime and who currently smokes.
Alcohol consumption: self reported habitual alcohol consumption.
Sample Size Calculations
The primary outcome variable of this study was incidence of VAP. Based on a previous study done in our institution which estimated the VAP at 43.4% [9], we calculated a total sample size of 146 with 80% power (5% significance level) to detect a 50% difference in incidence of VAP between the intervention and control group.
Statistical Analysis
All statistical analysis were performed using a standard software package (Stata, version 11.0; StataCorp). Descriptive statistics were obtained to summarise all baseline variables in each of the study groups. Analysis of the primary outcome variable involved comparing the rate of VAP between the two study groups. Simple unadjusted rates, relative risks and 95% Confidence intervals (CIs) were obtained. For other outcomes, a two sample t-test or Mann-Whitney test was used for continuous variables and chi-square tests were used for categorical variables. Analysis was based on the principle of ‘intention to treat’. Sensitivity analysis were done to determine the effect of missing data. All tests of significance were two-tailed.
Results
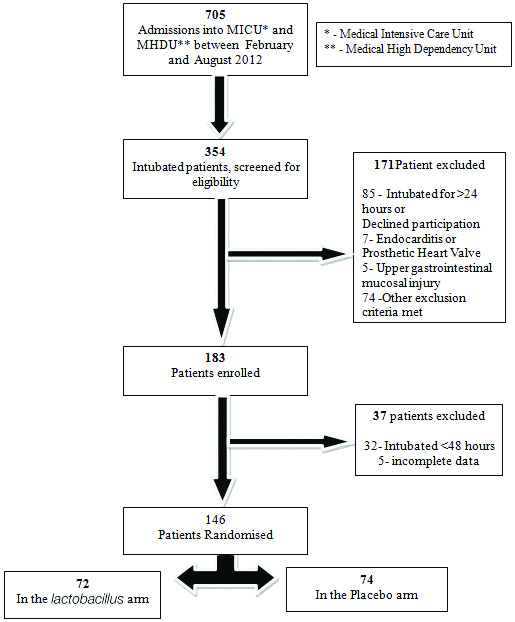
A total of 705 patients were admitted to the MICU and MHDU between February 2012 and August 2012. A total of 354 intubated patients were screened and 183 patients were included in the trial. The reasons for exclusion of the remaining 171 patients are given in [Table/Fig-1]. Of the 183 patients, a further 37 were excluded with reasons as mentioned. Hence, a total of 146 patients were included in the final analysis. A total of 72 of these patients were in the Lactobacillus arm and 74 in the placebo arm.
The baseline characteristics are shown in [Table/Fig-2]. Both groups had similar population characteristics except for alcohol consumption which was higher in the lactobacillus arm. The mean age, male/female distribution, APACHE II score, reason for admission and prevalence of co-morbidities were also similar in both groups. The mean APACHE II scores were 20 and 19 respectively in the Lactobacillus and placebo arm. The most common indications for admission were respiratory failure followed by poisoning in both arms. The most common setting for intubation was in the emergency department for both groups. The most common co-morbidities were diabetes and hypertension. Approximately, 15% of patients in both arms were on regular medications and these included anti-diabetic agents, anti-hypertensive, aspirin and statins among others. The median time to intubation was similar in both groups, 6 hours in the Lactobacillus arm and 5.7 hours in the placebo arm. A similar proportion of patients in both arms were on closed and open tracheal suction methods.
Baseline characteristics.
| Baseline characteristics | Lactobacillus arm N=72 | Placebo arm N=74 | p-value |
|---|
| Age (SD) | 42 (17) | 43 (17) | 0.73 |
| Male | 43 (60%) | 42 (57%) | 0.98 |
| Co-morbidities | N=38 | N=32 | 0.46 |
| Type 2 diabetes | 16 | 19 | |
| Hypertension | 16 | 21 |
| Current smoker | 8 | 5 |
| COPD | 5 | 6 |
| Alcohol consumption | 12 | 6 |
| Other respiratory disease | 5 | 3 |
| Reasons for admission* | | | 0.38 |
| Respiratory failure | 22 (31%) | 32 (43%) | |
| Haemodynamic support | 18 (25%) | 12 (16%) |
| Neurologic | 14 (19%) | 14 (19%) |
| Poisoning | 21 (29%) | 19 (26%) |
| Mean APACHE II **Score (SD) | 20 (8) | 19 (7) | 0.43 |
| Place of intubation | | | 0.28 |
| Emergency department | 29 (40%) | 27 (37%) | |
| On ICU arrival | 10 (14%) | 4 (5%) |
| During ICU stay | 14 (19%) | 18 (24%) |
| Ward | 7 (10%) | 11 (15%) |
| Previous hospital | 8 (11%) | 4 (5%) |
| Missing data | 4 (6%) | 10 (14%) |
| Median time to intubation (IQR) (hours) | 6 (0.15-35.5) | 5.7 (0.15-72) | 0.88 |
| Type of suction$ | | | 0.91 |
| Closed | 12 (17%) | 13 (18%) | |
| Open | 59 (82%) | 61 (82%) |
*Some patients had more than one reason for admission
**Acute Physiological, Age, Chronic Health Evaluation II Score
$Data missing for one patient in the Lactobacillus arm
Chi-square tests were used for comparison of frequencies; means were compared using independent-t-tests; and medians were compared using Mann-Whitney U test
Outcome Measures
Incidence of VAP [Table/Fig-3]
| Method of diagnosis of VAP* | Placebo arm N=74 | Lactobacillus arm N=72 | Total | p-value |
|---|
| Clinical criteria | 8 (11%) | 7 (10%) | 15 | 0.83 |
| Clinical criteria+CPIS **score | 7 (9%) | 6 (8%) | 13 | 0.91 |
*Ventilator associated pneumonia
**Clinical pulmonary infection score
Two-sample proportion test used
Of the 146 patients that were analysed, 15 patients developed VAP. Eight in the placebo arm (11%) and seven (10%) in the Lactobacillus arm- there was no statistically significant difference between the two groups.
When the more stringent CPIS was combined with the clinical criteria for the diagnosis of VAP, the incidence decreased to 8% and 9% in the Lactobacillus and placebo group respectively. Again there was no statistical difference between the two groups.
Early Versus Late VAP
The VAPs were categorised as early or late depending on the time to occurrence of VAP. Pneumonias that developed between 48 to 96 hours were considered as early VAP and those that occurred after 96 hours as late VAP. Of the total of 15 episodes of VAP, 11 (73%) were early VAP and 4 (27%) were late VAP. The incidence of early and late VAP between the two groups was similar.
ICU and Hospital Outcomes
Of the 72 patients in the Lactobacillus arm, 22 (31%) had an unfavourable outcome, that is, either died or discharged against medical advice, while the remaining 50 (69%) were discharged alive from the ICU.
The proportions were similar in the placebo group with 54 (73%) patients discharged alive and 20 (27%) either dead or discharged against medical advice.
With regard to hospital outcome 44 (61%) out of 72 patients in the lactobacillus arm were discharged alive while 28 (39%) had an unfavourable outcome (died or discharged against medical advice). Again the proportion of patients in the placebo arm was similar with 44 (59%) discharged alive and 30 (41%) with an unfavourable outcome.
Other Secondary Outcomes [Table/Fig-4]
| Timing of VAP | Lactobacillus arm | Placebo arm | p-value |
|---|
| Early VAP | 6 | 5 | 0.31 |
| Late VAP | 1 | 3 | 0.31 |
| Total | 7 | 8 | |
| ICU outcome | | | |
| Alive | 50 | 54 | 0.64 |
| Died / DAMA* | 22 | 20 | 0.64 |
| Hospital outcome | | | |
| Alive | 44 | 44 | 0.83 |
| Died/DAMA* | 28 | 30 | 0.83 |
| Other secondary outcome (days): |
| Median time to VAP (IQR) | 3 (2-5) | 3.5 (3-9) | 0.31 |
| Duration of ventilation (IQR) | 6 (4-8) | 7 (4-11) | 0.19 |
| Duration of ICU stay (IQR) | 7 (5-9.5) | 8 (6-13) | 0.11 |
| Duration of hospital stay (IQR) | 11.5 (7-19) | 14 (8-26) | 0.11 |
*DAMA: Discharged against medical advice
ICU: Intensive care unit
IQR: Inter quartile range
VAP: Ventilator associated pneumonia
Two-sample proportion tests for proportions; and Mann-Whitney U tests for comparison of medians
The other secondary outcomes analysed included median time to VAP, duration of mechanical ventilation, duration of ICU and hospital stay. None of the above secondary outcomes were significantly different between the two arms.
Microbiology of VAP
Out of the 146 patients evaluated, 15 developed a VAP. Two of these patients had no ET culture sent and in one, there was no growth on the ET culture that was sent. Hence a total of 12 patients had cultures that were positive. 26 different organisms were cultured on these 12 patients. Nine of these 12 patients had a poly-microbial VAP and the remaining three had mono-microbial VAP. The most common organism cultured was Non-fermenting gram negative bacillus (NFGNB) followed by Pseudomonas. There was no significant difference in the type of organism cultured in either group.
Antibiotic Sensitivity
Of the 26 organisms cultured, 16 were Carbapenem resistant and one was Colistin resistant. Five of the organisms cultured were sensitive to appropriate first line agents.
Discussion
The present trial did not show a significant difference in the incidence of VAP in those who received probiotic prophylaxis versus those who did not. Previous trials have shown conflicting results, but have been limited by difficulty in comparability between the trials. Three recent meta-analysis that evaluated the efficacy of probiotic prophylaxis in the prevention of VAP concluded that although there was a trend towards benefit, a strong recommendation for probiotics use could not be made. These studies were limited by clinical heterogeneity in trials, low quality evidence in some studies and a probable publication bias. They also concluded in each of their studies that further high quality clinical trials need to be conducted to conclusively establish the role of probiotics in the prevention of VAP [5,6,10].
This trial included 146 adult, MICU patients. The factors that contribute to the robustness of this study include that, it was a randomised study with appropriate allocation concealment, blinding of participants, their kin, health care providers and outcome assessors. There was no external funding for the study. All participants who were randomised were included in the final analysis and there was no attrition.
It has been observed that studies that have found probiotics useful in the prevention of VAP were usually done among surgical or trauma patients [11,12], and those done on medical patients usually yielded negative results- the findings in this study are consistent with that observation.
The rate of VAP noted in this study was also found to be less than that previously noted in our centre [9], this could be attributed to the strict adherence of VAP prevention bundles, increase in the awareness and positive changes in the overall infection control practice of the hospital staff and doctors. There was also no significant difference with regard to the timing of VAP (early versus late VAP).
In this study, the definition of VAP, was based on the clinical criteria by Johanson WG et al., [7]. The more stringent CPIS criteria were also used, but the incidence of VAP in either group was not significantly different regardless of the criteria for diagnosis. The administration of probiotics also did not result in improvement in any of the secondary outcomes also-median time to VAP, duration of mechanical ventilation, duration of ICU and hospital stay.
The most common organisms associated with VAP are gram negative bacteria [13,14]. In this study also, we found a similar result with NFGNB being the most common organism followed by Pseudomonas. Usually, it is expected that late VAP is associated with multi-drug resistant organisms while early VAP with organisms that are susceptible to first line antibiotics, however in this study we did not find such a difference and a majority of the organisms were multi-drug resistant. This reflects a changing trend in the incidence of multi-drug resistant pathogens that is now seen in critical care settings world over.
Importantly there were no cases of lactobacillus bacteraemia, once again pointing to the overall safety of probiotics in critically ill patients.
Limitation
The incidence of VAP in this study (11%) was much lower than the previous study (43.4%) which was used to calculate the sample size, the reason for this being the overall positive changes in hospital infection control policies and implementation.
Conclusion
The results of this randomised, placebo-controlled, double blinded study, done on 146, critically ill medical patients in the MICU of tertiary hospital in India show that probiotic administration did not have a significant effect on the prevention of VAP. Other outcome measures like ICU mortality, in-hospital mortality, median time to VAP, duration of mechanical ventilation and duration of ICU or hospital stay were also not influenced by the administration of probiotics. A large multicentre and randomised controlled trial which includes different critically-ill patient populations may be needed before we put this matter to rest.
*Some patients had more than one reason for admission
**Acute Physiological, Age, Chronic Health Evaluation II Score
$Data missing for one patient in the Lactobacillus arm
Chi-square tests were used for comparison of frequencies; means were compared using independent-t-tests; and medians were compared using Mann-Whitney U test