Since the late 1960s, conventional methods such as karyotyping are used for Prenatal Diagnosis (PD) of chromosomal anomalies. With advancement in technology, various prenatal invasive procedures such as CVS and amniocentesis were for detection of chromosomal anomalies such as aneuploidies, unbalanced and balanced structural anomalies. However, CVS testing is done in 10th week and amniocentesis are done in the 14th week of pregnancy possess high potential risk of miscarriages and development of fetal abnormalities. Based on existing data, these techniques have been known to result in 0.5% and 1% of cases of fetal loss respectively. Due to associated risk, these procedures have limited usage and screening tests currently used have limited confirmatory testing value. Therefore, as a result, it has become increasingly important to ascertain good confirmatory tests that are rapid, reliable, cost-effective and objective in their interpretation [1].
Samples obtained through invasive techniques can be analysed by various techniques to detect the presence of chromosomal abnormalities in fetus. Karyotyping is the gold standard analysis for detection of several conditions including mosaicism, polyploidy and chromosomal rearrangements such as deletions, translocation and inversions but its labour intensive nature, low resolution and a longer turnaround time limits its uses. These limitations have led to the development and utilisation of other techniques.
In 1990, FISH was developed to overcome conventional cytogenetics analysis, where the interphase cells remained undivided and therefore hindered rapid analysis of trisomy cases. FISH is based on specific fluorescent dye coupled to a probe complementary to a particular chromosome region; it targets only limited regions of the genome and are dependent on the viability of cells, specificity of probes and operator capability. Over the past years, quantitative QF-PCR is increasingly used for rapid diagnosis of aneuploidy in prenatal specimens [2-5]. Amplification of QF-PCR depends on Short Tandem Repeat (STR) markers generating a fluorescent product and is directly proportional to the amount of target sequence present in the initial template. This method is more applicable and cost-efficient than FISH [6-9].
In the present study, FISH and QF-PCR, collectively known as RAT, were compared in their ability to detect chromosomal aneuploidy of chromosome 13, 18 and 21 including sex chromosomes in prenatal samples of pregnant women with suspected fetal aneuploidies, since NIPT, CMA, whole Genome Sequencing (WGS) and Whole Exome Sequencing (WES) are costlier and their prescription depends mainly on clinicians choice.
Materials and Methods
This observational study was designed to compare two techniques i.e FISH and QF-PCR to investigate trisomic cases for rapid analysis.
Patient selection: Pregnant women as suggested by the clinician ranging in age from 20 to 46 years, suspected of having fetal aneuploidies were subjected to amniocentesis/CVS post approval and under the ambit of registered physicians.
These samples were analysed at Supratech Micropath Laboratory and Research Institute, Ahmedabad by QF-PCR and FISH. The CVS (12) and AF (108) samples after consultation were collected from different states of India and were tested during January 2016 -December 2017. For QF-PCR, predesigned chromosomal markers [Table/Fig-1,2] and specific FISH probes for trisomy 13, 18, 21 and sex chromosomes (Metasystem, Germany) were used.
Markers included in Aneufast-QF PCR for detection of chromosome 13, 18, 21, X and Y copy number.
| Marker | Label | Chromosome location | Known alleles (base pairs) |
|---|
| AMXY | 6-Fam | Xp22.1-22.31 - Yp11.2 | X 104 Y 109 |
| SRY | 6-Fam | Yp11.2 | Y 463 |
| X22 | 6-Fam | Xq28 Yq (PAR2) | 189-194-199-204-209-214-219-224-226-229-234-239-242-247-253 |
| DXYS218 | PET | Xp22.32 Yp11.3 (PAR1) | 266-270-274-278-282-286-290-294 |
| HPRT | 6-Fam | Xq26.1 | 264-268-272-276-278-280-284-288-292-296-300-313 |
| DXS6803 | VIC | Xq12-Xq21.33 | 106-110-114-118-120-124-128 |
| DXS6809 | VIC | Xq | 238-242-246-250-252-254-258-260-262-266-268-270-274 |
| DXS8377 | NED | Xq28 | 213-216-219-222-225-228-238-241-244-248-252 |
| SBMA | VIC | Xq11.2-Xq12 | 166-169-172-175-178-181-184-187-190-193-196-199-202-205-208-211 |
| D21S1414 | 6-Fam | 21q21 | 328-330-334-338-342-346-350-352-354-356-358-360-362-443 |
| D21S1411 | VIC | 21q22.3 | 246-262-266-274-278-282-286-290-294-298-302-306-316-319 |
| D21S1446 | PET | 21q22.3-ter | 200-204-208-212-214-218-220-224-228 |
| D21S1437 | VIC | 21q21.1 | 120-124-128-132-136-140-144 |
| D21S1008 | 6-Fam | 21q22.1 | 196-200-204-208-212-216-220 |
| D21S1412 | 6-Fam | 21q22.2 | 384-388-392-396-400-406-410-414-418 |
| D21S1435 | PET | 21q21 | 142-160-164-168-172-176-180-184-188 |
| D18S391 | VIC | 18pter-18p11.22 | 144-148-152-156-160-164-168 |
| D18S390 | VIC | 18q22.2 | 398-402-406-410-414-418-422-426-430 |
| D18S535 | NED | 18q12.2 | 126-130-134-138-142-146-148-152-156 |
| D18S386 | NED | 18q22.1 | 319-330-334-338-342-344-350-354-358-362-366-370-372-376-380-387 |
| D18S858 | PET | 18q21.1 | 186-190-192-196-200-204 |
| D18S499 | 6-Fam | 18q21.32-q21.33 | 386-392-396-400-404-408 |
| D18S1002 | 6-Fam | 18q11.2 | 122-130-134-138-142 |
| D13S631 | VIC | 13q31-32 | 192-196-200-204-208-212-215-218 |
| D13S634 | VIC | 13q14.3 | 460-464-466-470-474-478-482-484-486-490-496-500 |
| D13S258 | NED | 13q21 | 230-232-234-236-238-240-242-244-248-265-267-269-271-273-277-279-281 |
| D13S305 | PET | 13q12.1-13q14.1 | 426-430-434-438-442-446-450-454-458 |
| D13S628 | 6-Fam | 13q31-q32 | 436-440-444-448-452-456-460-464 |
| D13S742 | VIC | 13q12.12 | 254-258-262-266-268-270-274 |
Markers amplified with the Aneufast™ QF-PCR Kit the markers included in each of the six Primer sets are shown in the table.
| S1 | S2 | MXY | M21 | M18 | M13 |
|---|
| AMXY | SRY | SRY | D21S1411 | D18S386 | D13S631 |
| DXYS267 | X22 | AMXY | D21S1435 | D18S391 | D13S634 |
| D21S1414 | DXYS218 | HPRT | D21S1437* | D18S858* | D13S742* |
| D21S1446 | HPRT | TAF9L* | D21S1412* | D18S499* | D13S628* |
| D21S1442 | D21S1411 | DXYS156* | D21S1809* | D18S1002* | |
| D18S535 | D21S1435 | DXS6803* | | | |
| D18S391 | D13S634 | DXS6809* | | | |
| D18S976 | D13S258 | DXS8377* | | | |
| D13S797 | D18S386 | DXS981* | | | |
| D13S631 | D18S390 | DXS1187* | | | |
| D13S305 | | | | | |
*Additional markers for selected chromosome are added with the correspondent chromosome specific assays including two markers already present in the S1 and S2 assays to conform the identity of the sample.
The study was approved by Internal Ethical Committee of Gujarat University (IEC 001 GU) Ahmedabad.
DNA extraction: The DNA was extracted from CVS and Amniotic Fluid (AF) samples using a Qiagen DNA extraction kit. The kit was used according to the manufacturer’s instructions. The extracted genomic DNA was used as a template and was kept at 4°C until further use.
QF-PCR amplification and capillary electrophoresis: the amount of target sequence present in the initial template is directly proportional to QF-PCR amplification of STR markers that generates a fluorescent product.
The Aneufast™ QF-PCR Kit by Molgentix SL (Spain) with six multiplex marker sets of STRs was used for amplification of selected microsatellites and Amelogenin-SRY region of a chromosome. This combination of markers allowed the detection of aneuploidies involving chromosomes X, Y, 21, 18 and 13 with 100% sensitivity and specificity for non-mosaic trisomies. Two multiplex sets i.e S1and S2 of the kit were used to perform initial aneuploidy diagnosis and these assays were analysed through electrophoresis. In addition, four chromosome-specific marker sets (M21, M13, M18 and MXY) were used for the detection of trisomy 21, 13, 18 and sex chromosomes aneuploidies and as back-ups in case all the markers of S1 and S2 were non-informative (homozygous).
Five-dye DNA fragment analysis: The Aneufast™ QF-PCR kit (Molgentix, SL) is based on five-dye fluorescent system for automated DNA fragment analysis. Fluorochromes such as 6-FAM™, VIC™, NED™ and PET™ were used in conjunction with GS 500 LIZ™ size standard.
The data were analysed using GeneMapper IDX Version1.2 Applied Biosystems fragment analysis software following Aneufast QF-PCR kit (Spain).
Detection of trisomy 13, 18, 21 and sex chromosome: In a trisomic sample, three copies of a chromosome were detected by the presence of three peaks corresponding to chromosome specific STRs, having the same fluorescent intensity and a ratio between the areas of 1:1:1 (Trisomic Triallelic). In quantitative PCR the two chromosomes with the same repeat number, produced two unbalanced fluorescent peaks with an area ratio of 2:1 (Trisomic Diallelic). Triploid samples thus produced trisomic diallelic and triallelic patterns for informative STRs on all chromosomes.
FISH analysis: The trisomy of 13, 18, 21 and sex chromosomes were detected using probes i.e. X/Y D5608-100-OG; chromosome Y (Orange), chromosome X (Green); D5607-100-TC; for chromosome 13 (Blue), chromosome 18 (Green) and chromosome 21 (Red) as per FISH probe Metasystem protocols (Germany; catalogue 2017-2018).
Results
Screening tests: Of 120 patients screened, in case of CVS, one fetus showed absence of nasal bone. Ultrasound was also done for nuchal translucency in some cases, suggested by the Clinician [Table/Fig-3].
Trisomy screening result of amniotic fluid and chorionic villus sampling.
| Sr.nos/Sample types | Abnormality | Gestational age (Weeks) | Maternal age (Years) | Screening markers | Nasal bone | NT (mm) | FISH result normal | FISH result abnormal | QF-PCR result normal | QF-PCR result abnormal |
|---|
| 1. Amniotic fluid (108) | Normal (104)46 XY/XX | 12-20 | 27 | Double/triple/Quadruple | - | | 104 | - | 104 | - |
| Abnormal trisomy 18 (2)47,+18 | 17. 116. 5 | 42/28 | Double (+ve)Quadruple (+ve)NIPT (+ve) | Present/- | 1.9/- | - | 04 | - | 04 |
| Abnormal trisomy 21 (2)(47,+21) | 19. 2/15.2 | 35/27 | Double (+ve) Triple (+ve)NIPT (+ve) | Present/- | 3.2/ | - | - | - | - |
| 2. CVS (12) | Normal (11)(46 XY/XX) | 8 -12 | 27 | Double/triple/Quadruple | - | - | 11 | - | 11 | - |
| Abnormal trisomy 13 (1)47 + 13 | 12.5 | 27 | Double (+ve) | Absent | 2.5 | - | 01 | - | 01 |
CVS=Chorionic villus sampling; AF=Amniotic fluid; NIPT=Non invasive prenatal testing; +ve=Positive; NT=Nuchal translucency; QF-PCR=Quantitative Fluorescence-Polymerase Chain Reaction; FISH=Fluorescent in situ hybridization Total cases 120; Trisomy total=5/120 (4.2%); AF 4/108 (3.7%) and CVS=1/12 (8.3%). Figures in Parentheses indicate sample type analysed; RAT=Rapid Aneuploidy Testing
FISH Analysis revealed, four abnormal signals, two samples depicted trisomy of chromosome 18 (blue dots) and two samples depicted trisomy of chromosome 21 (red dots), constituting 3.7% (4/108) aneuploidy in amniotic fluid samples. Among 12 CVS samples, only one trisomy of 13 (Patau) case (green dots) was detected (1/12; 8.3%) [Table/Fig-4a, 5a, 6a].
Results of FISH (a) and QF-PCR (b) for detection of trisomy of 18.
| Techniques | Trisomy-18 |
|---|
| FISH | 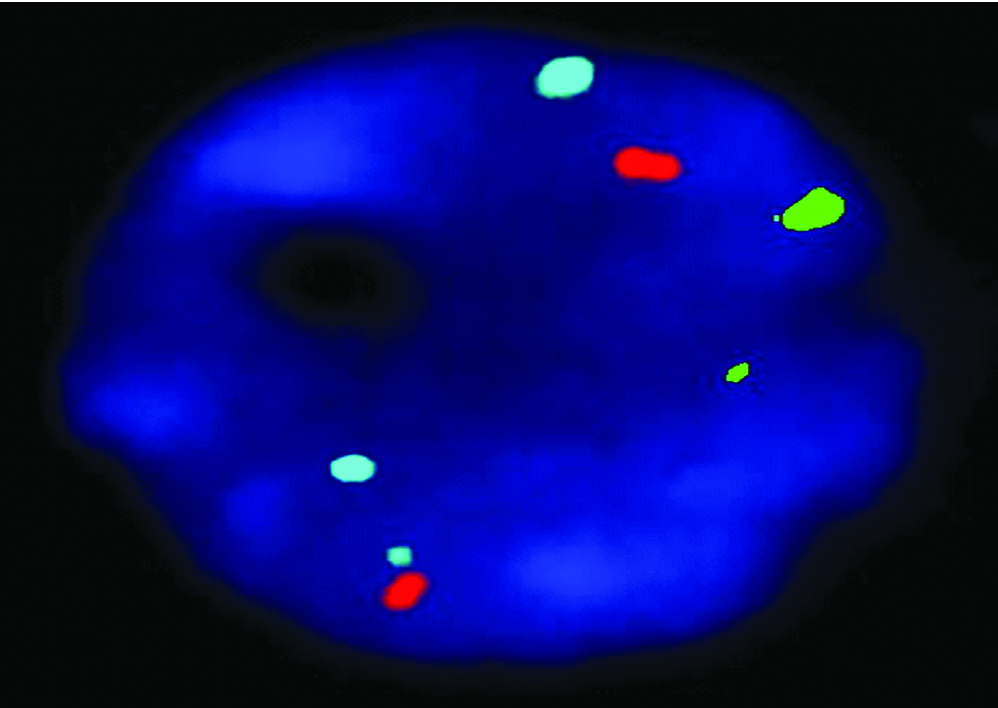 |
| QF-PCR | 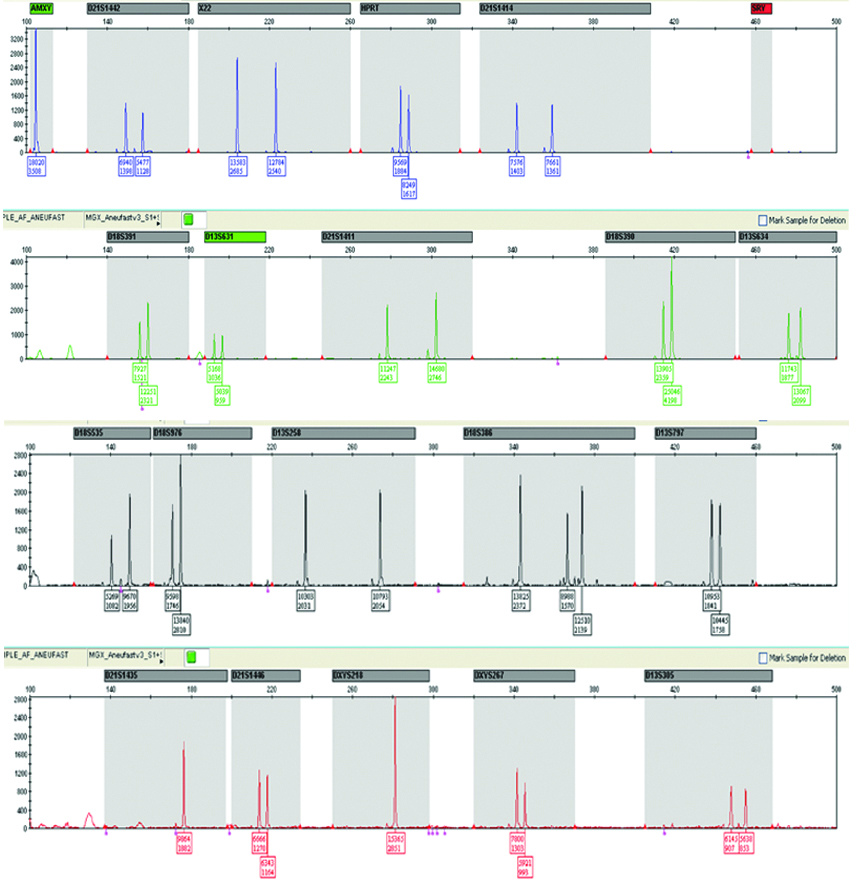 |
Results of FISH (a) and QF-PCR (b) for detection of trisomy of 21
| Techniques | Trisomy-21 |
|---|
| FISH | 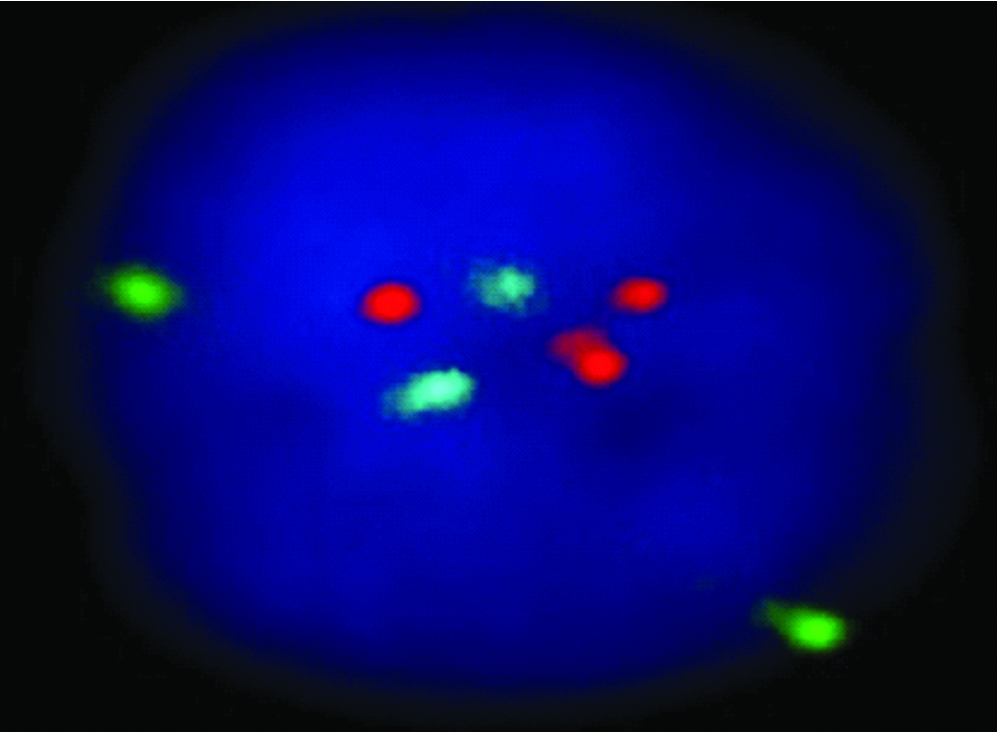 |
| QF-PCR | 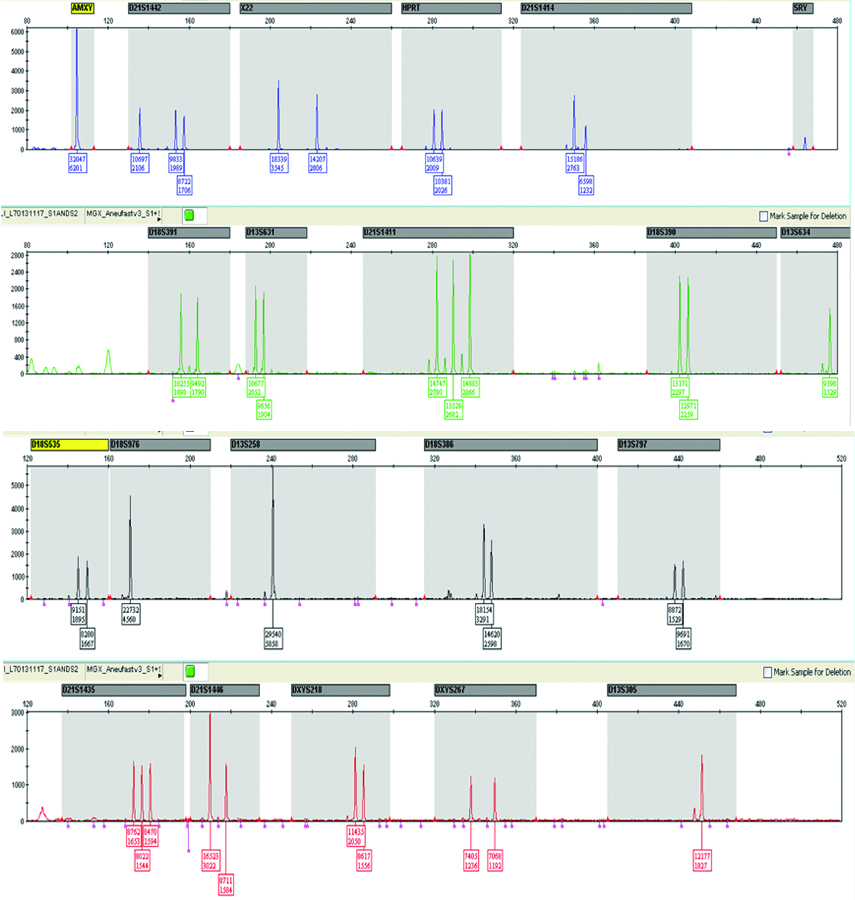 |
Results of FISH (a) and QF-PCR (b) for detection of trisomy of 13.
| Techniques | Trisomy-13 |
|---|
| FISH | 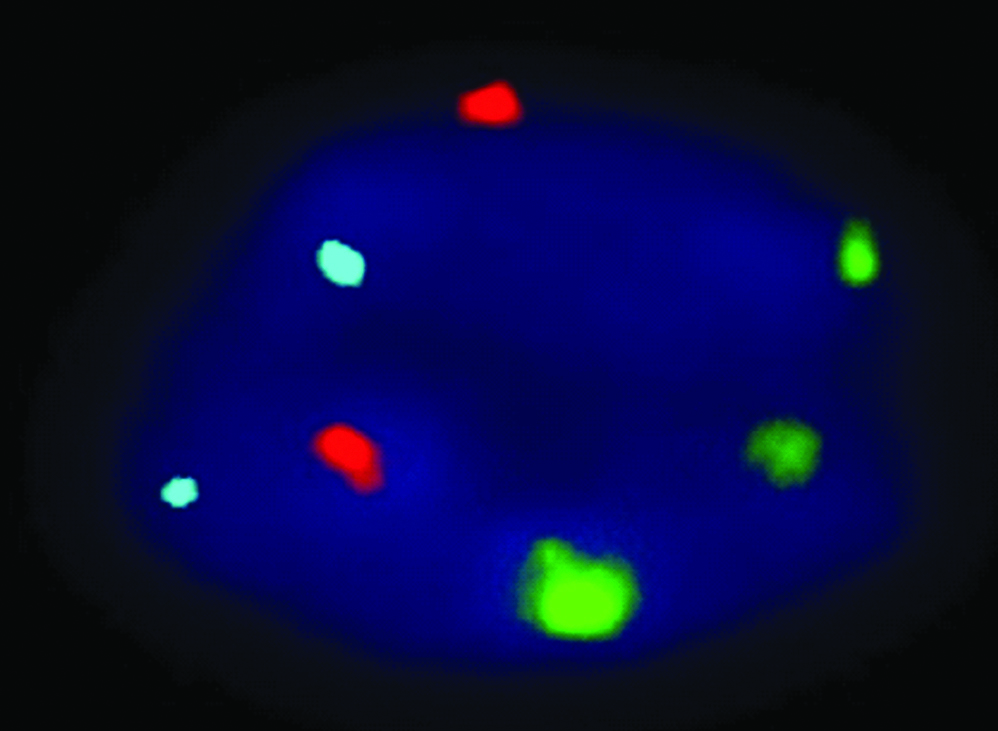 |
| QF-PCR | 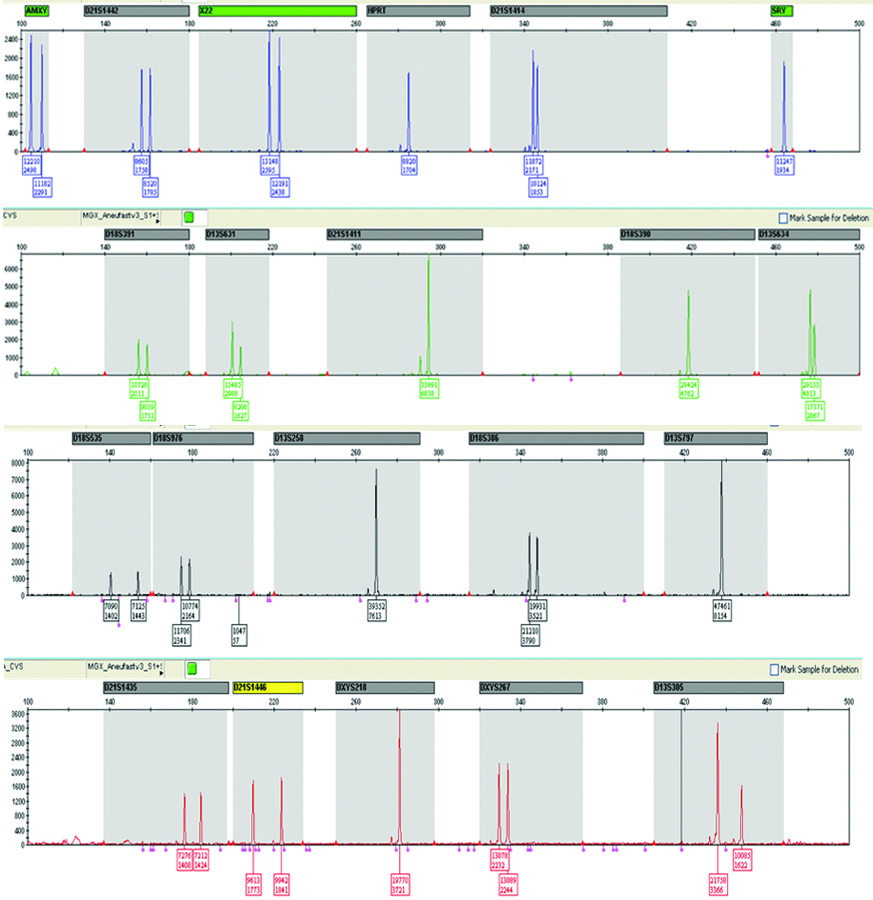 |
QF-PCR: Two samples showed trisomy of 21 and three homozygous STRs peaks of same intensity and same area ratio 1:1:1 called trisomy triallelic was observed. In trisomic aneuploidy of chromosome 18 and 13, two unbalanced which fluorescent peaks are ratio of 2:1 called trisomic diallelic was detected [Table/Fig-4b, 5b, 6b]. In the present study, no sex chromosomal trisomy was observed.
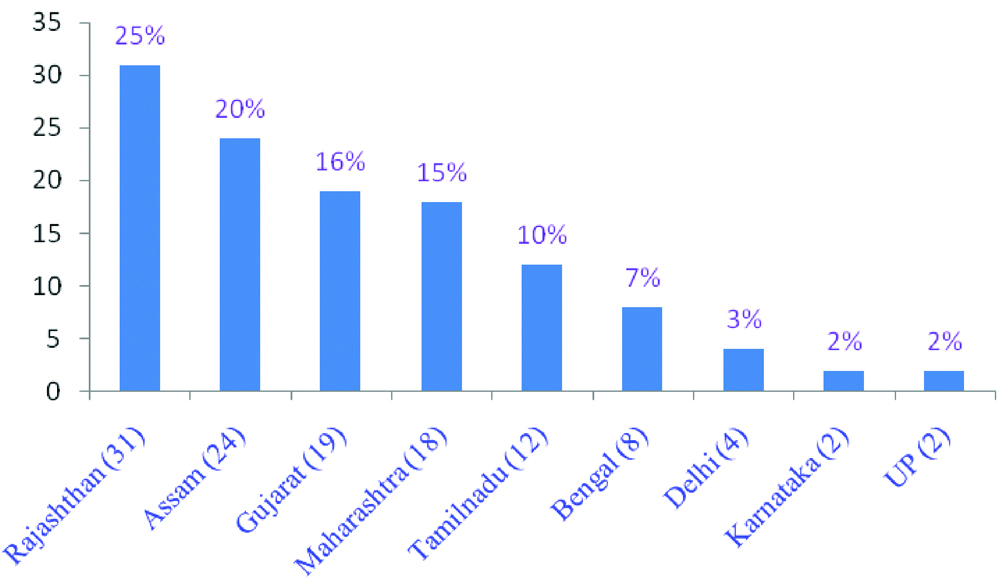
Geographic pattern: Out of 120 AF and CVS samples, higher frequency was obtained from Rajasthan followed by Assam, Gujarat and other states of India [Table/Fig-7].
State-wise sample distribution of aneuploidy study.
Discussion
In this cohort, RAT jointly FISH and QF-PCR were used to detect common aneuploidies as an adjunct to karyotyping. The results suggest that FISH could possibly be replaced by QF-PCR for prenatal aneuploidy detection where skilled manpower or cytogenetics infrastructure are crucial. The Association of Clinical Cytogeneticists (ACC) reported that 1% of all prenatal samples are likely to have a chromosomal abnormality which remains undetected sometimes. [10]. While, NIPT, A non-invasive prenatal test using cell free fetal DNA (cffDNA) has very high sensitivity and specificity for T21, it is slightly sensitivity for T13 and T18 detection. Therefore, NIPT should not be used for total diagnosis [11,12]. Insufficient fetal fraction and high associated cost makes it unsuitable for retesting [13]. And 25% of invasive diagnostic procedures could be avoided [14]. In the present study cohort, 120 referral samples including AF and CVS were used for detecting aneuploidies during pregnancy. Given that these samples were collected from a wide geographic distribution across India, a variety of initial screening tests i.e. double, triple, quadruple and ultrasound were done for advance maternal age patients (35-42 years) based on physician’s preference. To understand the geographic distribution of the Indian states in regard to these genetic disorders, the samples from various parts of India such as Rajasthan (25%), Assam (20%) followed by Gujarat (16%) and Maharashtra (15%) were analysed [Table/Fig-7].
Samples that were found to be positive in initial screening techniques were then processed by RAT i.e. FISH and QF-PCR, where the former is laborious, expensive and limited whereas the latter is fast, relatively cheaper and takes only approximately 24 hours for testing [6,14-18].
The clinical application of RAT was applied in the present study for prenatal diagnosis of aneuploidy samples comprising of AF and CVS that were analysed after initial screening tests. The results obtained by both techniques were comparable. Various authors such as Leung WC et al., Badenas C et al., and Gross S et al., reported that RAT is a reliable technique for detection of only trisomy of chromosome 13, 18, 21, and sex chromosome trisomy were detected when ultrasound detected anomalies were consistent suggesting that RAT does not detect all chromosomal abnormalities that would be picked up by traditional gold standard method karyotyping [4,14,15,18-20]. Similar findings were reported, and aneuploidy testing using invasive FISH and QF-PCR tests were recommended [16].
Among these techniques, FISH is direct examination of intact cells which is advantageous but is relatively expensive and laborious process. And QF-PCR should be prefered for detection of polyploidy STRs and prenatal rapid aneuploidy detection [5,17-22].
Limitation
The sample size was relatively small and conventional karyotypic analysis of the samples was not done. Further study with larger sample size are required to establish the utility of RAT for detection of aneuploidies. In addition, studies are required to determine the efficacy of RAT in detection of sex chromosome aneuploidy.
Conclusion
In this report, the data suggest that for rapid diagnosis of aneuploidy, QF-PCR is better in terms of cost as well as turnaround time compared to Fluorescent In-Situ Hybridization (FISH) and conventional karyotyping. For errorless results Rapid Aneuploidy Test (RAT) is suggested, though non-invasive (NIPT, NIPS) and invasive (CMA, WES, WGS) tests are available for diagnosis, that are expensive and therefore are not very commonly prescribed.
*Additional markers for selected chromosome are added with the correspondent chromosome specific assays including two markers already present in the S1 and S2 assays to conform the identity of the sample.
CVS=Chorionic villus sampling; AF=Amniotic fluid; NIPT=Non invasive prenatal testing; +ve=Positive; NT=Nuchal translucency; QF-PCR=Quantitative Fluorescence-Polymerase Chain Reaction; FISH=Fluorescent in situ hybridization Total cases 120; Trisomy total=5/120 (4.2%); AF 4/108 (3.7%) and CVS=1/12 (8.3%). Figures in Parentheses indicate sample type analysed; RAT=Rapid Aneuploidy Testing