T2DM is one of the most common chronic diseases with increasing prevalence trend throughout the world [1]. According to reports of World Health Organisation (WHO), globally 422 million (9%) of adult had diabetes in 2014 and this figure was expected to increase to 642 million by 2040 [2]. The disease is one of the leading cause of mortality particularly premature deaths, disabilities, and health care expenditures [3]. In 2015, 1.6 million deaths occurred directly due to the toT2DM and economic burden of the disease was estimated to be 1.3 trillion US dollars [3]. In Iran, T2DM is one of the important chronic diseases. According to the diabetes atlas, about 5 million (9.6%) of Iranians aged 20-79 year suffered from T2DM in 2017 [4]. Due to the significant impacts of the disease including mortality, various morbidities, and financial burden and decreasing quality of life, T2DM is regarded among the most important public health problems in the world [3,5].
Lifestyle modification and pharmacotherapy are basic components to control hyperglycemia for T2DM management [6]. In this regard, Medication Adherence (MA) is an important aspect of T2DM management [6,7]. MA is defined as the degree to which a patient follows a medication regimen [8]. To adhere to medications, the drugs should be taken according to the prescribed dosage, dosing interval, duration of treatment and other related recommendations [7,8]. MA is necessary for blood glucose control, preventing or delaying diabetes-related complications, and decreasing costs [9].
One of the most widely used self-report methods for measuring the MA level, particularly in chronic diseases, is the MMAS-8 [10-12]. This tool has been extensively applied for assessment of drug-taking behaviour in various types of diseases especially chronic conditions [11]. Different psychometric properties of this questionnaire have been reported in several studies in Malaysia, Korea and China for different types of diseases [13-15]. In three studies among T2DM patients, the Cronbach’s alpha for MMAS-8 was reported as 0.67, 0.65, and 0.66 and test-retest reliability value was expressed as 0.79, 0.80, and 0.71 [12,13,15]. The result of a meta-analysis revealed that MMAS-8 had acceptable internal consistency and test-retest reliability for assessing MA in T2DM patients [16]. The MMAS-8 is a short and simple questionnaire that can be used for research and clinical purposes. Owing to its advantages, it has been broadly used for measuring treatment adherence in a wide range of acute and chronic physical and mental disorders [17-19].
Studies among Iranian hypertensive patients with Persian version of MMAS-8 have shown contradictory results about validity and reliability of the scale [20,21]. The authors found no study about assessing psychometric properties of Persian MMAS-8 among T2DM patients in the first level of health delivery system of Iran. The current study was aimed to investigate the validity and reliability of Persian version MMAS-8 among T2DM patients in healthcare centres of Kerman, Iran.
Materials and Methods
Participants, Setting and Design
A cross-sectional study was conducted among T2DM patients from January to March 2017. The studied population included T2DM patients in urban healthcare centres of Kerman city, Iran. Out of 43 urban health Centres affiliated to Kerman city, 12 Centres were selected via random samplling method. Next, 50 patients from each of the 12 selected Centres were enrolled in the study through a convenience samplling method. Therefore, the studied sample included 600 participants. The inclusion criteria were T2DM patients with at least one year of disease duration and at least one-year usage of antidiabetic medications (oral antidiabetic agents or insulin). To assess the test-retest reliability, 30 patients participated to complete the MMAS-8 in two-time points with an interval duration of 10 to 14 days.
Instruments
The English version of MMAS was translated to the Persian language by two qualified translators. After comparing two translations by one of the researchers, backward translation to the English language was done. Finally, an expert panel consisting of the researchers and the translators confirmed the final Persian version of MMAS-8. The questionnaire consists of 8 items. Each item assesses a specific aspect of medication-taking behaviour and is not a determinant of MA. Items 1 to 7 have binary yes/no response and Item 8 is recorded in a 5-point Likert’s scale (“never”, “rarely”, “sometimes”, “usually”, and “all the time”). For scoring the yes/no responses, “no” and “yes” responses were scored as 1 and 0, respectively; except for the Item 5 that scoring was done in reverse. For Item 8 “never” to “all the time” responses were rated as 0, 0.25, 0.5, 0.75, and 1, respectively [16,21].
Also, the present authors used a Diabetes Self-Management Questionnaire (DSMQ) in the study. This questionnaire evaluates the self-care behaviour of diabetic patients such as diet, physical activity, blood glucose testing, and drugs taking behaviours [22]. The subscale of MA of this questionnaire was used to assess the convergent validity of MMAS-8. The MA subscale has two items: 1) I take my diabetes medication (e.g., insulin and tablets) as prescribed; 2) I may forget to take or skip my diabetes medication (e.g., insulin and tablets). The answers of the items were recorded in a 4-Likert scale (“does not apply to me”, “applies to me to some degree”, “applies to me to a considerable degree” and “applies to me very much” scored as 0, 1, 2, and 3, respectively). The score of MA subscale was calculated as the summed score of the items and then transformed to a score ranging from 0 to 10 [22,23].
Data Collection and Ethical Considerations
The questionnaires were completed by conducting face-to-face interviews with the eligible patients. Data collection for all the participants was done by a trained interviewer. Prior to the interview, the interviewer explained the objectives of the study to the participants and ensured them about the confidentiality of the collected data. Also, after obtaining the written consent the patients enrolled in the study. The patients who did not accept to enrol in the study received diabetes care services as same as the participants in the study. Furthermore, the study proposal was approved by the ethics committee of Kerman University of Medical Sciences (Ethical Code: IR.KMU.AH.REC.1396.1301).
Statistical Analysis
Cronbach’s alpha method was used to measure internal consistency. ICC was used to check the test-retest reliability. The subscale of MA of DSMQ was also used to assess the convergent validity of MMAS-8. To determine the correlation convergent validity, correlation analysis method was applied. EFA by the Principal Component Method (PCM) was performed for 300 people out of all randomly selected participants. Component and rotated component were considered to determine the factors of MMAS-8. Factor loadings lower than 0.4 were omitted. Also, CFA was run on 289 remaining participants. The present authors used p-value, CFI, Tucker-Lewis Index (TLI), Root Mean Square Error of Approximation (RMSEA), and Normal Fit Index (NFI) to report the model fit. Furthermore, CFA was performed using the maximum likelihood estimation method. In all the analyses, p-value <0.05 was considered as a criterion of statistical significance. All statistical analyses were accomplished using SPSS 20.0 including AMOS 23.0.
Results
Data of 589 participants out of 600 was used for data analysis (response rate 98.1%). The mean (±SD) age of the participants was 56.40 (±11.9) year and more than 72% (n=426) belonged to the age group of 64 years or younger. Just above two-third (67.9%, n=400) of the participants were women and 32.1% (n=189) were men. Over half (51.1%, n=301) of the patients had high school education, followed by 38% (n=224) primary school or illiterate and 10.9% (n=64) university education. More than 73% (n=423) of the subjects were married and 55.7% (n=328) were housewives. The mean scores of MMAS-8 and MA subscale of DSMQ were 6.27 (95% CI: 5.91-6.63) and 8.07 (95% CI:7.60-8.53), respectively.
Total Cronbach’s alpha for MMAS-8 on 30 participants was 0.75, which showed good internal consistency. Removing any items of the instrument did not result in a significant change in alpha value. ICC was 0.88, indicating good test-retest reliability. The correlation coefficient between the MMAS-8 and MA subscale of DSMQ was 0.85 (p<0.0001), which was a good convergent validity.
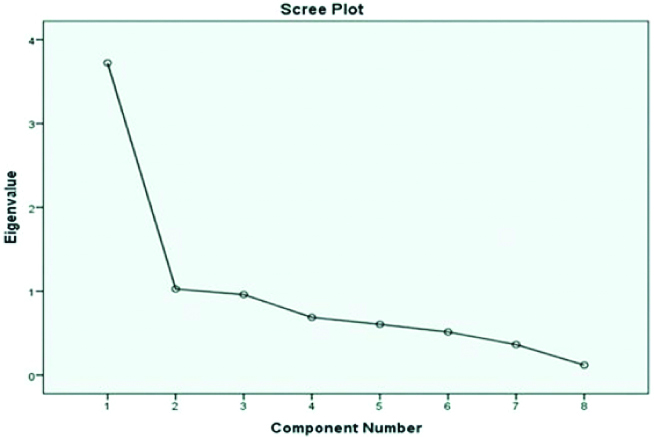
Kaiser-Meyer-Olkin, which is a measure of samplling adequacy, was equal to 0.8 for the instrument used in this study. The p-value of Bartlett’s test of sphericity was statistically significant (p<0.0001), suggesting that EFA was useful for the present data. As presented in [Table/Fig-1], EFA for 300 participants revealed two factors. The first factor included Items 1, 2, 3, 4, 6, and 8. This dimension had an eigen value of 3.72 and accounted for 46.5% of the total variance. Also, Items 5 and 7 fell in the second dimension, which had an eigenvalue of 1.02 and accounted for 12.8% of the total variance. The scree plot indicated that the eigenvalues of the first two dimensions were greater than 1, and the eigenvalues tended to be equal after the third factor [Table/Fig-2]. The present authors reported the frequency and percentage of responding for the 8 items. In addition in all questions, the frequency of “yes” responses was considerably less than “no” responses except for Items 5 and 7.
Exploratory factor analysis, component and rotation component of initial factor extraction of MMAS-8 Persian version in type 2 diabetic patients using the principal component method.
| | Yes response | No response | Component | Rotated component |
|---|
| Item | | N (%) | N (%) | Factor 1 | Factor 2 | Factor 1 | Factor 2 |
|---|
| 1 | | 127 (42.3) | 173 (57.7) | 0.79 | - | 0.8 | - |
| 2 | | 76 (25.3) | 224 (74.7) | 0.79 | - | 0.78 | - |
| 3 | | 46 (15.3) | 254 (84.7) | 0.76 | - | 0.76 | - |
| 4 | | 57 (19) | 243 (81) | 0.65 | - | 0.65 | - |
| 5 | | 285 (95) | 15 (5) | - | 0.58 | - | 0.59 |
| 6 | | 49 (16.3) | 251 (83.7) | 0.66 | - | 0.66 | - |
| 7 | | 146 (48.7) | 154 (51.3) | - | 0.8 | - | 0.79 |
| 8 | Never/RarelySometimesUsuallyAll the time | 165 (55)88 (29.3)42 (14)5 (1.7) | | 0.91 | - | 0.91 | - |
Scree plot obtained from exploratory factor analysis.
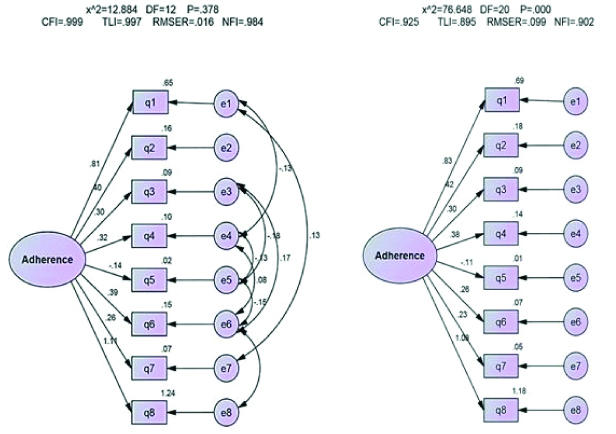
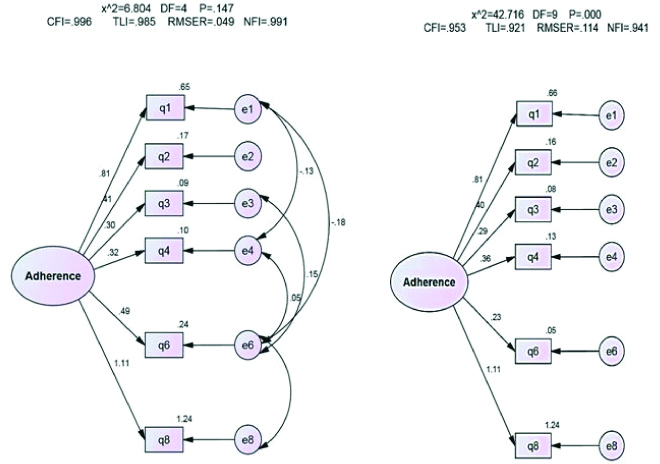
The present author performed CFA of 289 participants in two scenarios. In the first and second scenarios, also assessed CFA for all 8 items and six items (without Items 5 and 7, which fell in factor 2), respectively [Table/Fig-3,4]. All indices, as presented in [Table/Fig-5], were acceptable for modified MMAS with 8 items (χ2=12.88, DF=12, p=0.37, CFI=0.99, RMSEA=0.016, TLI=0.99, and NFI=0.98) as well as for modified MMAS without items 5 and 7 (χ2=9.3, DF=6, p=0.15, CFI=0.99, RMSEA=0.04, TLI=0.98, and NFI=0.98). Also, unmodified model for MMAS with 8 items (χ2=76.64, DF=20, p=0.0001, CFI=0.92, RMSEA=0.1, TLI=0.89, and NFI=0.90) and MMAS without items 5 and 7 (χ2=42.7, DF=9, p=0.0001, CFI=0.95, RMSEA=0.11, TLI=0.92, and NFI=0.94) are reported in [Table/Fig-2]. As can be seen, unmodified model P and RMSEA indices were not acceptable.
Modified and unmodified models for MMAS-8 with 8 item. Data are standardised regression coefficients for path arrows. All regression coefficients are significant with p <0.001.
Modified and unmodified models for MMAS-8 without items 5 and 7. Data are standardised regression coefficients for path arrows. All regression coefficients are significant with p <0.001.
Confirmatory factor analyses of MMAS-8, testing modified and unmodified models with 8 item and without items 5 and 7 (Random sample n=289).
| | X2 | DF | P | CFI | RMSEA | TLI | NFI |
|---|
| Modified | MMAS with 8 item | 12.88 | 12 | 0.37 | 0.99 | 0.016 | 0.99 | 0.98 |
| MMAS without items 5 and 7 | 6.80 | 4 | 0.14 | 0.99 | 0.049 | 0.98 | 0.99 |
| Unmodified | MMAS with 8 item | 76.64 | 20 | <0.001 | 0.92 | 0.1 | 0.89 | 0.90 |
| MMAS without items 5 and 7 | 42.70 | 9 | <0.001 | 0.95 | 0.11 | 0.92 | 0.94 |
Discussion
The aim of this study was to report the reliability and validity of the Persian version of the MMAS-8 among T2DM patients in the first level of healthcare delivery system of Iran. The results of the current study showed acceptable internal consistency of the scale and an excellent test-retest reliability. Besides, convergent validity between the MMAS-8 and MA in the DSMQ questionnaire was high enough. Also, construct validity was checked using EFA and CFA. EFA demonstrated two distinct factors that were confirmed by CFA, as well. To the best of authors knowledge, this study was the first attempt to determine psychometric properties of the Persian version of the MMAS-8 in T2DM patients. However, there are some studies to assess the Persian version of MMAS-8 among hypertensive patients in Iran [20,21,24]. In the present study, Cronbach’s alpha was 0.75, which is consistent with previous studies in Asian countries such as China, Pakistan, Saudi Arabia, and Turkey [25-28]. Moreover, this finding is in line with the results of some studies from European countries such as Poland and Greece [29,30]. Cronbach’s alpha for MMAS-8 in other languages is between 0.4 and 0.81 but it is higher than 0.7 in many other studies [25-30]. Cronbach’s alpha value of the present study is consistent with many other studies on the non-English version of MMAS-8, which has a lower Cronbach’s alpha compared to the original version of the scale (0.83). In a study conducted using the Persian version of MMAS-8 among hypertensive patients, Cronbach’s alpha was about 0.7, which is consistent with the present results. However, in another study among Iranian hypertensive patient, Cronbach’s alpha was 0.4 [20,21].
The present study demonstrated that the MMAS-8 had a good test-retest reliability (ICC=0.88), which is consistent with the studies conducted in Thailand, Indonesia, Brazil, China, and Pakistan, in which ICC was >0.8 [15,27,31-33]. In two studies conducted among Iranian hypertensive patients, test-retest reliability was reported as 0.89 and 0.94, which are the same as the present result. Hence, it can be stated that the reliability of the Persian version MMAS-8 is acceptable.
The current study revealed a high positive correlation (r=0.85, p<0.001) between MMAS-8 and MA subscale of DSMQ. In the original study for development and validation of the scale, the correlation between MMAS-8 and MMAS-4 was 0.64. Studies on patients with T2DM in Malaysia, Thailand, and Pakistan reported a correlation between MMAS-8 and another version of Morisky scale (MMAS-4 and MMAS-3) as 0.79, 0.77, and 0.76, respectively [13,27,32]. The high correlation of MA subscale of DMSQ with MMAS-4 and MMAS-8 can be attributed to using the same items in these instruments. The results of the present study revealed two factors for the MMAS-8. Factor 1 included items 1, 2, 3, 4, 6, and 8. Xu M et al., stated that Factor 1 represents general non-adherent behaviours and Jankowska-Polanska B et al., argued that this factor is related to patients’ behaviours in the past [29,34]. Moreover, Items 5 and 7 fell within Factor 2. In a study in Spain among psychiatric outpatient setting and another study in Uganda among hypertensive patients, Item 7 (“Do you ever feel hassled about sticking to your antidiabetic treatment plan?”) fell within Factor 2 [35,36]. In both studies, it was reported that Item 7 is about the emotion of patients but other items deal with medication taking behaviours that reflected an attitude towards medication. Some studies revealed that item 5 (“Did you take all your anti-diabetic medicines yesterday?”) could be placed in Factor 2. For example, some studies in Poland, China, Iran, and Uganda among patients with different diseases have shown that Item 5 falls in factor 2 [21,29,34,36]. An explanation for this result may be that, Item 5 asks about a behaviour on the previous day, but in other items, the occurrence of behaviours is measured during a longer period [35-36]. In other studies, similar to the presents study, Items 5 and 7 show different results from those of other items [17,26]. Factor loadings for Items 5 and 7 were respectively 0.42 and 0.5 in a survey to assess validation of Turkish version of MMAS-8 among hypertensive patients [26]. Moreover, in a study to evaluate the original version of MMAS-8, Items 5 and 7 had the lowest factor loading compared to the rest items [17].
Limitation
In this study, for the first time, the present authors assess the psychometric properties of MMAS-8 among T2DM patients in Iran. Despite the advantages of this work, it also had some limitations. First, there was no Haemoglobin A1c level in medical records of the majority of the participants for assessing the criterion validity. The second limitation was that the participants of this study (i.e., patients who attended in the healthcare Centres) could not exactly represent all diabetic patients.
Conclusion
The Persian version of MMAS-8 has acceptable reliability and validity for assessing MA among T2DM patients. MMAS-8 is a brief and simple scale to measure MA. So, it can be applied for Iranian diabetic patients by health care providers and researchers. Furthermore, the current study has identified that items 5 and 7 of the MMAS-8 were not in the same direction as other items. Hence, the present authors suggest conducting further studies to change, revise, or omit items 5 and 7 for enhancing the construct validity of MMAS-8.