Colorectal Cancer (CRC) is the third most common cancer in terms of incidence and the second leading cause of cancer death worldwide [1]. The CRC incidence increased by 34% between 2006 and 2016, but 19% and 12% of this was because of ageing and growing populations, respectively, and only 2% is thought to be due to changes in the age-specific incidence rate [1]. Although the highest incidence has been reported from some parts of Europe, there have been decreases in both incidence and mortality rates in United States and France. In contrast, countries undergoing major development transitions have been faced with increasing trends of both CRC incidence and mortality, which point to the influence of westernisation of the populations, changes in lifestyle, obesity, and dietary habits on CRC aetiology [2]. In Iran, CRC is currently the 2nd most common type of cancer and the 4th leading cause of cancer mortality [3]. According to last results of East Azerbaijan population based cancer registry, CRC was the second most common cancer incident in both sexes, with an Age Standardised Incidence Rates (ASIRs) of 18.2 and 13.7 per 100,000 males and females respectively [4].
According to the latest American and European clinical practise guidelines, it is mandatory to evaluate the genotype of tumour tissues for KRAS and NRAS mutations in mCRC before initiation of anti-EGFR targeted therapies [8-10]. However, some data have demonstrated that BRAF mutations also reduce the treatment benefit from targeted therapies and are a significant marker of a poor prognosis in CRC. Therefore, testing mCRCs for BRAF mutations has been recommended recently as a prognostic and survival prediction biomarker [8]. Different gene mutations may confer different degrees of biological aggressiveness and reduce the effectiveness of targeted therapy strategies [11].
Materials and Methods
Study Design and Data Collection
This study was a cross-sectional analytical study, and all mCRC cases referred to two main central hospitals and oncologists’ clinics, in Tabriz from January 2016 to November 2018 were enrolled.
We evaluated the data of 280 cases, while the inclusion criteria were with histologically confirmed CRC with any metastasis confirmed by imaging (CT scan and/or MRI). From these we retrieved 173 cases that met our inclusion criteria and mutation tests were performed.
In most of the cases, available mutation tests were based on a referring oncologist’s recommendation. For some others after getting signed consent forms for performing the mutation tests, we used the patients’ Formalin-fxed paraffn-embedded (FFPE) tissue samples. We excluded the cases where we couldn’t find the samples and/or patients. Characteristics of sex, age at diagnosis, grade, and family history of CRC, tumour histological type, Body mass index (BMI), primary tumour location, and any history of smoking and alcohol consumption were collected.
Tissue Sample Collection and Molecular Tests
After confirmation of mCRC, while an expert pathologist undertook histological examination of the cancer tissues, additional FFPE tissue sections (5-30 μm) from the samples underwent molecular tests for KRAS, NRAS, and BRAF mutations in the reference molecular laboratory. According to the latest clinical guidelines established in the oncology Centres of Tabriz, KRAS, NRAS, and BRAF mutation tests were performed routinely for all mCRC cases before considering any treatment strategies. About 67% of the cases were based on a referring oncologist’s recommendation, and for the some others after getting signed consent forms for performing the mutation tests, we used the patients’ samples.
Mutations were detected by the Idylla KRAS/NRAS/BRAF Biocartis NV system (2800 Mechelen, Belgium, BCT006631). According to the reference clinical guidelines [14], for KRAS gene, these mutations have been detected for codons 12, 13, 59, 61, 117, 146: Gln61His (c.183G>C), Gly12Ala (c.35G>C), Gly12Asp (c.35G>A), Gly12Cys (c.34G>T), Gly12Cysl (c.34G>T), Gly12Ser (c.34G>A), Gly12Val (c.35G>T), Gly13Asp (c.38G>A), and G13D.
NRAS mutations detected included Q61R (c.182A>G), and for BRAF was V600E/D (c.1799T>A). The Turnaround Times (TAT) from sample shipping to getting the mutation results were under 10 days for >95% of present cases. Three to four FFPE tissue sections (5-30 μm) of CRC tumours were loaded within the cartridges, and then inserted in the system according to manufacturer instructions. After the DNA extraction and purification by the system, multiplex- PCRs were performed, and finally KRAS/NRAS/BRAF specific software automatically determined the presence of mutations.
The Ethics Committee of Local University of Medical Sciences has approved this project (Code: IR.TBZMED.REC.1395.18), and all patients information and records are confidential.
Statistical Analysis
The present authors used STATA MP 14.2 (StataCorp LP, College Station, Texas 77845 USA) for data analysis. To detect relationship of clinicopathologic characteristics with each of the mutations logistic regression models were used. Odds ratios (ORs) of sex, age, and histological type, positive family history of CRC, BMI, anatomical subside, grade, smoking, alcohol consumption, and presence of mutations were analysed. Unadjusted and adjusted ORs along with 95% Confidence Intervals (CIs) were presented. Mutation status was considered as dependent variable, and mutants were compared with wild-type genes.
Results
Study Subjects
Among 173 enrolled CRCs, 97 patients were men (56.1%) and 76 cases were women (43.9%), with a mean age of 58.65 (±13.67) years. About 75% of the patients were older than 50 years (n=130) and adenocarcinoma was the most common histological type (86.1%, n=149). The mean BMI was 24.94 (±4.79) with a range of 11.90 to 42.40. There were 30 patients who had a history of smoking (17.3%) and 8.7% (n=15) of the patients had consumed alcohol during the last 10 years. About 15.6% (n=27) of the patients had a positive family history of CRC (at least in one of their first degree relatives).
Mutation Characteristics
Complete mutation analysis data were available for KRAS in 102 cases, for NRAS in 75 cases, and for BRAF in 39 cases. Among 102 patients with KRAS gene detection data, the frequency of mutations was 38.23% (n=39/102). Among the mutations, most were in exon 2, codon 12 (61.54%, 24/39), followed by 12.82% (5/39) in codon 13 and 1 case in codon 61 (2.56%). Codon 12 was affected by 6 mutational types, and the most frequently observed mutations were Gly12Val (c.35G>T) and Gly12Asp (c.35G>A). NRAS mutations were only observed in 1 patient (1.33%) from among the 75 cases who were tested for NRAS mutations; this mutation was in codon 61 with the mutational type Q61R (c.182A>G). BRAF codon 600 was tested in 39 cases, and only one case (2.56%) had a mutation with the mutational type of V600E/D (c.1799T>A). The mutations and their frequencies are summarised in [Table/Fig-1].
Mutation distribution in metastatic colorectal cancer patients.
| Mutation | Frequency | Percent (%) |
|---|
| KRAS |
| Gln61His (c.183G>C) | 1 | 2.56 |
| Gly12Ala (c.35G>C) | 1 | 2.56 |
| Gly12Asp (c.35G>A) | 8 | 20.51 |
| Gly12Cys (c.34G>T) | 2 | 5.13 |
| Gly12Cysl (c.34G>T) | 1 | 2.56 |
| Gly12Ser (c.34G>A) | 3 | 7.69 |
| Gly12Val (c.35G>T) | 9 | 23.08 |
| Gly13Asp (c.38G>A) | 4 | 10.26 |
| G13D | 1 | 2.56 |
| Unknown | 9 | 23.08 |
| Total | 39 | 100 |
| NRAS |
| Q61R(c.182A>G) | 1 | |
| BRAF |
| V600E/D (c.1799T>A). | 1 | |
Relationship between Mutations and Clinicopathological Aspects
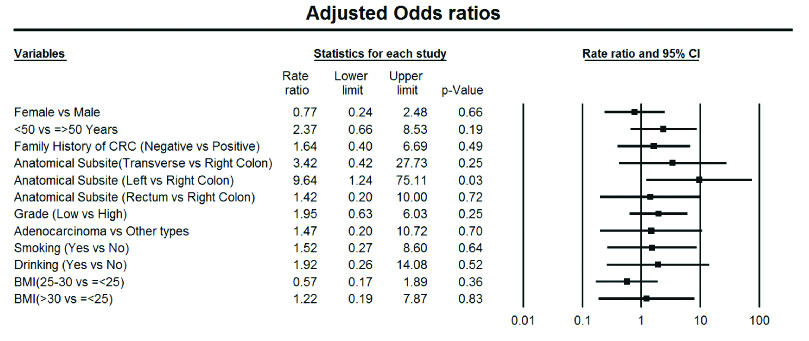
Regression analysis was performed for evaluation of any association of age, sex, tumour location, grade, histological type, BMI, smoking, and alcohol consumption with KRAS gene mutations. This analysis was not performed for the NRAS and BRAF mutations because of their low frequencies. Younger patients (<50-year-old) had significantly about 3-fold higher odds of having KRAS mutations, and adenocarcinoma histological type increased the odds of KRAS mutations about 2.5 times compared with any other histological type. Smoking and alcohol consumption increased the likelihood of KRAS mutation. Tumour location had a strong impact on the likelihood of KRAS mutation; as compared with right-sided tumours, left-sided tumours had 10 times (OR=10.00; 95% CI=2.15–46.47) and rectal tumours 2 times (OR=2.00; 95% CI=0.48–8.26) higher odds of KRAS mutations, and this association was statistically significant for left-sided tumours (p=0.003). Positive family history of CRC decreased the likelihood of KRAS mutation, while the odds of mutation was 1.71 times higher in patients with negative family history (OR=1.72; 95% CI=0.60-4.89), but this was not statistically significant. After adjustment of all of the mentioned variables (age, sex, grade, morphological type, smoking, alcohol consumption, BMI, tumour location), Multi-variate regression analysis showed that only the location of the tumour had a significant impact on the likelihood of KRAS mutation. Patients with left-sided tumours had about 9.5 times higher likelihood of mutation than right-sided tumours (OR=9.64; 95% CI=1.24–75.27) (p=0.031). The results of the un-adjusted and adjusted regression analysis were summarised in [Table/Fig-2,3].
Results of unadjusted and adjusted regression analysis for association of kras mutation and clinicopathological aspects (Uni-variate and Multi-variate Binary Logistic Regression).
| Variable | KRAS mutation | Univariate regression | Multivariate regression |
|---|
| Mutant (39) | Wild type (63) |
|---|
| Number (Percent) | Number (Percent) | OR | 95% CI | p-value | OR | 95% CI | p-value |
|---|
| Lower | Upper | Lower | Upper |
|---|
| Sex | Male (97) | 21 (53.8%) | 35 (55.6%) | Ref | - | - | - | Ref | - | - | - |
| Female (76) | 18 (46.2%) | 28 (44.4%) | 1.07 | 0.48 | 2.39 | 0.866 | 0.77 | 0.24 | 2.49 | 0.664 |
| Age | <50 (43) | 17 (43.6%) | 13 (20.6%) | 2.97 | 1.23 | 7.16 | 0.015 | 2.37 | 0.66 | 8.55 | 0.187 |
| ≥50 (130) | 22 (56.4%) | 50 (79.4%) | Ref | - | - | - | Ref | - | - | - |
| Family history of CRC | Negative (146) | 33 (84.6%) | 48 (76.2%) | 1.72 | 0.60 | 4.89 | 0.310 | 1.64 | 0.40 | 6.66 | 0.489 |
| Positive (27) | 6 (15.4%) | 15 (23.8%) | Ref | - | - | - | Ref | - | - | - |
| Anatomical subsite | Right colon (21) | 3 (7.7%) | 14 (22.2%) | Ref | - | - | - | Ref | - | - | - |
| Colon transverse (52) | 9 (23.1%) | 14 (22.2%) | 3.00 | 0.67 | 13.47 | 0.152 | 3.42 | 0.42 | 27.61 | 0.249 |
| Left colon (36) | 15 (38.5%) | 7 (11.1%) | 10.00 | 2.15 | 46.47 | 0.003 | 9.64 | 1.24 | 75.27 | 0.031 |
| Rectum (64) | 12 (30.8%) | 28 (44.4%) | 2.00 | 0.48 | 8.26 | 0.338 | 1.42 | 0.20 | 9.91 | 0.723 |
| Grade | Low grade (66) | 21 (53.8%) | 22 (34.9%) | 2.17 | 0.96 | 4.91 | 0.062 | 1.95 | 0.63 | 6.02 | 0.247 |
| High grade (107) | 18 (46.2%) | 41 (65.1%0 | Ref | - | - | - | Ref | - | - | - |
| Histological types | Adenocarcinoma (149) | 35 (89.7%) | 53 (84.1%) | 2.64 | 0.53 | 13.18 | 0.236 | 1.47 | 0.20 | 10.63 | 0.704 |
| Others (14) | 2 (5.1%) | 8 (12.7%) | Ref | - | - | - | Ref | - | - | - |
| Unknown (10) | 2 (5.1%) | 2 (3.2%) | - | - | - | - | - | - | - | - |
| Smoking | Yes (30) | 7 (17.9%) | 11 (17.5%) | 1.07 | 0.38 | 3.04 | 0.903 | 1.52 | 0.27 | 8.64 | 0.636 |
| No (141) | 31 (79.5%) | 52 (82.5%) | Ref | - | - | - | Ref | - | - | - |
| Unknown (2) | 1 (2.6%) | - | - | - | - | - | - | - | - | - |
| Drinking | Yes (15) | 6 (15.4%) | 6 (9.5%) | 1.75 | 0.52 | 5.89 | 0.366 | 1.92 | 0.26 | 13.98 | 0.521 |
| No (154) | 32 (82.1%) | 56 (88.9%) | Ref | - | - | - | Ref | - | - | - |
| Unknown (4) | 1 (2.6%) | 1 (1.6%) | - | - | - | - | - | - | - | - |
| BMI | ≤25 (64) | 20 (51.3%) | 30 (47.6%) | Ref | - | - | - | Ref | - | - | - |
| 25-30 (36) | 10 (25.6%) | 24 (38.1%) | 0.63 | .25 | 1.58 | 0.322 | 0.57 | 0.17 | 1.87 | 0.352 |
| >30 (17) | 3 (7.7%) | 6 (9.5%) | 0.75 | 0.17 | 3.35 | 0.160 | 1.22 | 0.19 | 7.91 | 0.837 |
| Unknown (15) | 6 (15.4%) | 3 (4.8%) | - | - | - | - | - | - | - | - |
OR: Odds ratio; CI: Confidence interval; CRC: Colorectal cancer; BMI: Body mass index
Results of multivariate regression analysis, for association of KRAS mutation and Clinicopathological Aspects showing adjusted Odds Ratios with 95% CI.
Discussion
Colorectal cancer (CRC) is the second most common cancer in Iran and in East Azerbaijan as well [4], with increasing trend of incidence in recent decade in the country [13,15]. The present study report a cohort of metastatic CRC patients from the Azeri ethnic population in the Northwest of Iran. Among patients enrolled over three years present assessed the KRAS, NRAS, and BRAF mutational distribution using the Idylla KRAS/NRAS/BRAF molecular diagnostic system along with the main clinicopathological aspects of the mCRCs. The overall frequency of KRAS mutations was 38.23% (n=39). NRAS mutation was detected in only 1 patient (1.33%) among 75 cases who were tested, and among 39 cases who were tested for BRAF mutations only 1 case (2.56%) had a mutation, V600E/D (c.1799T>A).
While the frequency of KRAS mutation in mCRC patients is 24.0% in Asian populations, the overall frequency of KRAS mutation in our study was higher (38.23%) [6,16,17]. The present results for KRAS mutations were similar to a few reports from European studies, where the frequency reported was about 36.0% [7,11,18], but authors found a lower frequency of NRAS and BRAF (1.33 and 2.56% respectively) mutations. According to the results of the TRIBE retrospective evaluation, KRAS, NRAS, and BRAF mutations were detected in 52.8, 5.3, and 7.5%, respectively [19]. In another study from Spain, the mutation tests identified NRAS and BRAF mutations in around 22% of mCRCs [7]. Also, a previous study on CRC patients revealed that 26% of CRC cases had a heterozygote-mutant KRAS and mutations were not detected in the amplified exon of BRAF [20]. The main reason for these differences is still unclear. Molecular test methodology and quality, different mechanisms of gene and environmental interactions, and genetic heterogeneity and ethnicity maybe the main reasons for this difference [18].
Similar to the present results, mutation analysis in Japan showed that 37.4% of mCRC patients had KRAS mutations [21]. However, in the present study, the most prevalent KRAS mutation type was within codon 12, Gly12Val (23.08%), while mutations within codon 13 were the most prevalent type (29.7%) in their study, with an amino acid change of Gly13Asp, which is similar to the results of Sirisena ND et al., from Sri Lanka with a frequency of 40% [6]. However, they found a higher frequency of Gly12Asp amino acid changes (27.0%), within codon 12, which is the second-most frequent mutation type in the present CRCs (20.51%) after Gly12Val (23.08%) [21].
The present authors found a significant association between clinicopathological aspects and the likelihood of KRAS mutation, while patients with left-sided tumours had about 9.5 times greater likelihood of mutation than right-sided cases, so tumour location had impact on the likelihood of KRAS mutation, according to the present results. There have been conflicting results about any relationship between the presence of KRAS mutations and clinicopathological aspects. Some studies have demonstrated no significant differences between the presence of KRAS mutation and variables such as age, sex, tumour location, and histological type [6]. However, some studies have revealed different results. In a study from China, Bai B et al., showed that KRAS mutations were significantly more common in women and mutations were common in right-sided tumours compared with left-sided ones [17]. According to the present results, left-sided tumours had a significantly greater likelihood of KRAS mutation, but sex was not significantly correlated with the presence of mutation. In another study from China, Pang XL et al., reported that KRAS mutations were not correlated significantly with sex, histological type, or grade; however, they found that in older CRC patients, KRAS mutations were significantly more common [16], while the present authors found that younger mCRC patients had significantly higher odds of KRAS mutation.
However, there are challenges of the high-cost and lack of availability of anti-EGFR therapies in Iran and the necessity of developing the robust KRAS, NRAS, and BRAF mutation tests, so many oncologists strongly recommended to selecting the most appropriate cases for targeted therapy treatments in order to avoid unnecessary costs in every local context [6,13]. Also, detection of oncogenic mutations, which are associated with the prognosis and clinical outcome of CRC patients, maybe useful for monitoring disease progression and assisting in early diagnosis of metastatic disease [11]. Prospective studies using highly sensitive KRAS/NRAS/BRAF analysis will be useful in early detection of high risk individuals for metastasis, and may promote the development of more effective treatment interventions, to select the mCRC patients who might benefit from targeted therapies (anti-EGFRs) [22].
Limitation
One of the main limitations of this study is high-costs of molecular tests and so low sample size. However, we could find a few NRAS/BRAF mutations among the present patients, which may be because of small sample size or molecular techniques.
Conclusion
This study tried to evaluate the oncogenic mutation pattern in mCRC as a cohort survey for the first time in East Azerbaijan. The present authors found some interesting results about association of clinicopathologic aspects and KRAS mutations. The overall frequency of KRAS mutations in the present study was high, while the frequency of KRAS mutations in mCRC patients is lower in Asian populations. KRAS mutation results in this study were most similar to results of European populations.
Authors Contribution
Conception and designing was done by RD, SD; Administrative support was given by IAK, JEZ, AN, ZS; Provision of study material or patients was conducted by: ATES; Collection and assembly of data was done by RD; Data analysis and interpretation were done by: RD, SD; Manuscript was written by: RD, SD, MHS; Final approval of manuscript was done by RD, SD, IAK, JEZ, AN, ZS, ATES, MHS.