Intestinal Parasitic Infections (IPIs) are a serious public health problem in most of the regions of the world, especially in developing countries and represent a major cause of morbidity and mortality in children [1]. According to global estimates, it has affected nearly 3.5 billion people and caused morbidity in about 450 million. Globally, Ascaris lumbricoides causing ascariasis, Trichuris trichiura causing trichriasis and Hookworms (Ancylostoma duodenale & Necator americanus) causing helminthiasis are among the most common IPIs, presenting with symtoms of abdominal pain, diarrohea, nausea, vomiting, fatigue, etc., [2]. In India, the overall prevalence rate of IPIs ranges from 16.5% to 66% [3]. In India, Ascaris lumbricoides is the most common parasite followed by Hookworm, Hymenolepis nana, Tapeworm, Trichuris trichiura, Enterobius vermicularis, Entamoeba histolytica, Entamoeba coli and Giardia lamblia [2]. The prevalence of different IPIs depends upon the environmental, social, individual, community and economical factors [1]. There are many morbidities which are caused due to helminthic infections namely anaemia, malnutrition, vitamin A deficiency, etc.
Tuberculosis (TB) continues to be one of the most important causes of morbidity and mortality worldwide. Based on the WHO report in 2015, globally around 10.4 million people developed pulmonary TB (PTB) and 1.4 million people died from all forms of TB [4]. “TB Statistics India– National, Treatment outcome and State statistics” estimated TB burden in the year 2016 as incidence of TB in India 211 per one lac population per year. In Odisha, the incidence was 195 per one lac per year, with a sputum positivity rate of 54% [5].
Both IPIs and TB are chronic infectious diseases, causing serious harm to humans [6]. The geographic distribution of helminths and TB overlap substantially, increasing the likelihood of co-infection with both the pathogens [7].
During active TB, asymptomatic helminth infection is associated with increased regulatory T-cell and Th2-type response; helminth infection is also significantly correlated to a reduced rate of sputum smear positivity [8]. Co-infection of TB and intestinal parasites hastens progression of the disease and increases morbidity in PTB patients [6].
Inspite of the increased morbidity caused by co-infection, data about the infection rate of IPIs among PTB patients is lacking in this part of eastern India. Therefore, this study was planned with an objective to determine the prevalence of IPIs among PTB patients, to find out the type of IPI in them and to analyse the association of risk factors with the prevalence of co-infection.
Materials and Methods
This hospital based cross-sectional study was conducted in a tertiary care teaching hospital, Bhubaneswar, Odisha, India; between July 2015-Sept 2015.
Study population: All patients attending the outpatient (OPD) and in-patient (IPD) of the Pulmonary Medicine Department, who were clinically diagnosed as newly suffering from PTB, with the following inclusion and exclusion criteria:
Inclusion criteria: Both OPD and IPD newly diagnosed patients of PTB who gave written informed consent and willingness to participate in the study, patients aged one year and above and of both sexes.
Exclusion criteria: Individuals who had already started anti-TB treatment, participants who had taken anti-parasitic drugs two weeks prior to the specimen collection, un-cooperative patients who expressed their unwillingness to come for follow-up for the study procedure were excluded.
Ethical clearance: The study was approved (Ref. No.:KIMS/KIIT/IEC/32/2016, dated 27.05.2016) by Institutional Ethics Committee, of the medical college.
Sample size: In the study period a total of 118 patients were enrolled for our study.
Study procedure: The OPD and IPD cases attending the Pulmonary Medicine Department, who were clinically diagnosed as newly suffering from PTB and confirmed by either Ziehl-Neelsen [ZN] staining of sputum smear for Acid-Fast Bacilli (AFB), radiological evidences or both, were approached. Written informed consent was taken from those above 18 years and from the parents of the minor children, explaining the purpose of the study and assuring their confidentiality. The study tool comprised of a proforma with details of name, sex, age, status of the disease, past history of TB and other relevant data.
Three stool samples were collected from each participant. The first sample was collected during first interview and the follow-up second and third samples were collected on 2nd and 3rd day for confirmation from IPD patients. OPD patients were given two containers with proper instructions to provide two more stool samples on successive visits.
Collection of stool samples: For proper collection of sample and for the detection and identification of intestinal parasites, a small screw capped plastic bottle with wooden scoop was provided to each patient who were included in the study. They were instructed to fill half the bottle and discard the scoop after use. Instructions were given to take precautions before collection of feces, like not to mix urine with stool sample. The samples collected were brought to the laboratory for processing. All the containers along with specimen were properly labeled.
Examination in the laboratory: Macroscopic examination of the fecal sample was done for color, consistency and presence or absence of blood, adult parasites or exudates. Microscopic examination was done by both direct wet mount method and formalin-ether concentration method. In both cases saline-wet mount and iodine mount preparations were done and observed for parasitic infections by using a binocular microscope under 10X and confirmed by observing under 40X magnification [9]. Fresh and concentrated stool specimens were examined as saline wet mounts to detect motile trophozoites, larva, cysts and ova of protozoan parasites.
Reporting of gross and microscopic features of stool was done and presence of any parasitic infection if diagnosed was considered after being confirmed by a second observer.
Statistical Analysis
Data collected was entered into Microsoft excel worksheet and analysed using Epi Info Software (version 3.5.4). Descriptive statistics was used for presentation of data; statistical significance was calculated using chi-square and F-test as appropriate with a p-value of <0.05 considered to be statistically significant.
Results
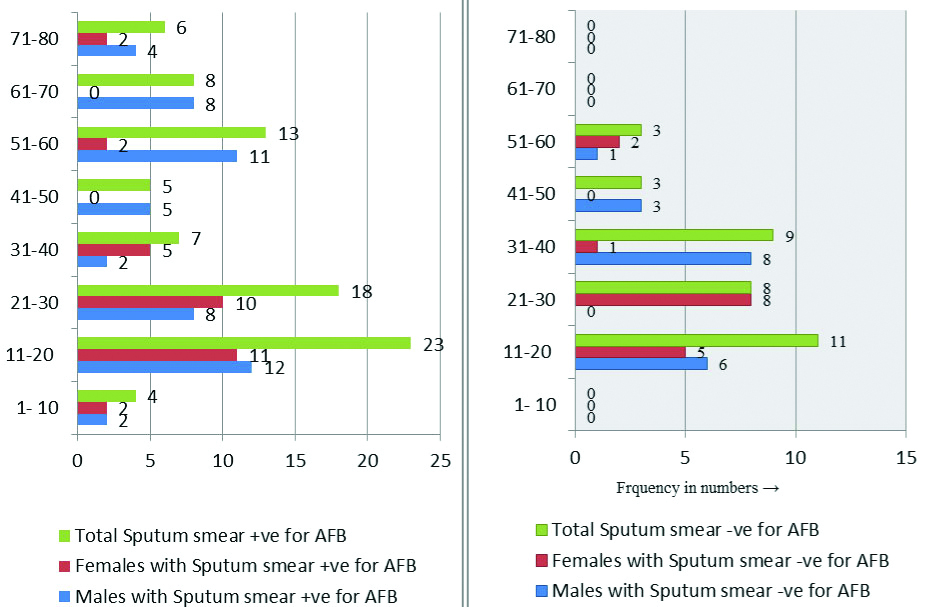
Out of 118 TB patients enrolled in the study, 84 (71.18%) were sputum smear positive, 34 (28.81%) were smear negative for AFB by ZN stain [Table/Fig-1]. The prevalence of intestinal infections among the study group was 27.11%.
Age and sex wise distribution of pulmonary TB patients (n=118).
Smear positive PTB cases Smear negative PTB cases (n=84) (n=34)
Socio-Demographic Characteristics
Around 28.81% of the participants were in the 11-20 year age group followed by 22.03% in the 21-30 year age group [Table/Fig-1]. A 59.3% were males; 19.49% were literates and majority (72.03%) belonged from rural areas [Table/Fig-2].
Socio-demographic characteristics of the pulmonary TB patients (n=118).
| Socio-demographic variables | Intestinal parasitic Infections | p-value |
|---|
| Present (n=32) | Absent (n=86) |
|---|
| Age group (in years) |
| 1-15 (n=38) | 15 | 23 | 0.11 |
| 16-45 (n=50) | 10 | 40 |
| >45 (n=30) | 7 | 23 |
| Sex |
| Male (n=70) | 20 | 50 | 0.82 |
| Female (n=48) | 12 | 36 |
| Literacy status |
| Illiterate (n=95) | 26 | 69 | 0.89 |
| Literate (n=23) | 6 | 17 |
| Place of residence |
| Rural (n=85) | 23 | 62 | 0.84 |
| Urban (n=33) | 9 | 24 |
A 27.37% of those who were illiterate and 26.09% of those literate had intestinal parasitic co-infection. A 27.06% of the PTB patients from rural and 27.27% of those from urban areas had intestinal co-infection. However, it was seen that none of the variables like age, sex, literacy status and place of residence had statistically significant association with intestinal infection status [Table/Fig-2].
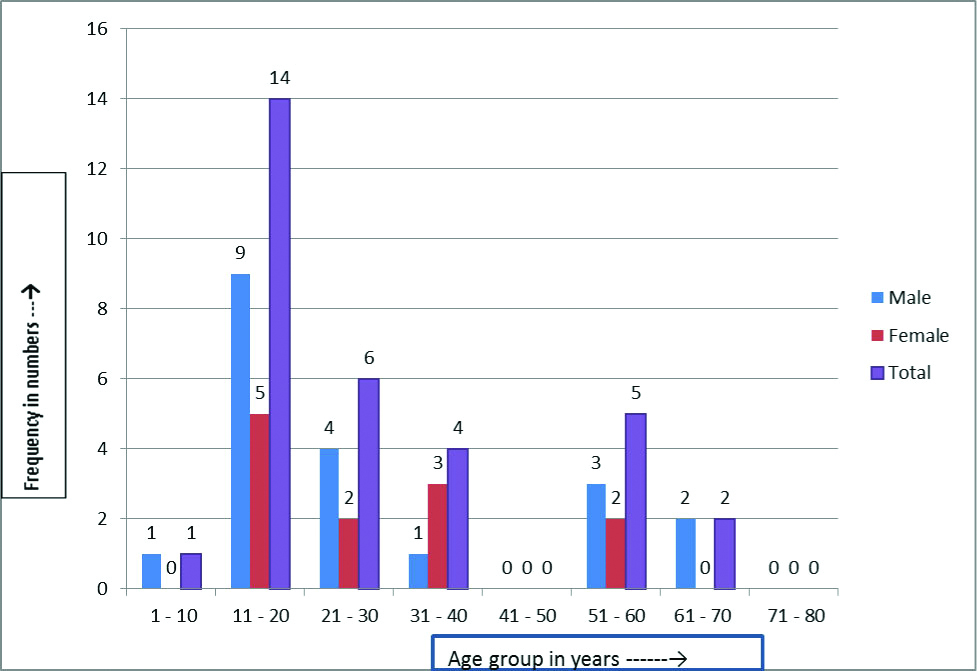
In this study, the prevalence of IPIs in smear positive cases was 30.95% (26/ 84) and smear negative cases was 17% (6/ 34). A 3.1% of those in less than ten years age group were having intestinal infections and all were males. Maximum (43.75%) of those having intestinal infections were in the age group of 11-20 years (64.28% of them were males and 35.72% females). A 75% of those in 31-40 years age group were females [Table/Fig-3]
Age and sex distribution of intestinal parasitic infections in pulmonary TB cases (n=32).
While analysing different types of parasitic infections in the study group, it was found that out of 32 cases with IPIs, 28 (87.5%) were having helminths and 4 (12.5%) protozoal infections [Table/Fig-4]. Hookworm (A. duodenale and N. americanus) infection was found to be the most common parasite accounting for 65.63% of the total parasitic positive cases. On analysis with respect to sputum AFB status, it was found that, 22.62% smear positive cases had hookworm co-infection in comparison to 5.88% smear negative cases and this was also found to be statistically significant.
Co-Infection of different intestinal parasites in pulmonary TB Patients (n=118).
| Parasites | Status of sputum smear for AFB (n=118) | p-value |
|---|
| +VE (n=84) | -VE (n=34) |
|---|
| Helminths | Hookworm | 19 | 2 | 0.02 |
| Strongyloides stercoralis | 5 | 0 | 0.32 |
| Ascaris lumbricoides | 2 | 0 | 0.92 |
| Protozoa | Entamoeba histolytica | 0 | 4 | 0.0001 |
Prevalence of Strongyloides stercoralis and Ascaris lumbricoides in this study was found as second and third common helminth infection i.e., 5/32 (15.6%) and 2/32 (6.25%) respectively with no significant association with smear-positive status.
In the present study, co-infection with Entamoeba histolytica was the only protozoal infection found. None of the smear-positive patients were suffering from Entamoeba histolytica co-infection whereas 11.74% of those who were smear-negative had the same. This difference was also found to be highly statistically significant.
Discussion
In this hospital based cross-sectional study, done with the objectives to find out the prevalence of intestinal parasitic co-infection, among 118 newly diagnosed laboratory and/or radiologically confirmed PTB cases, from July to September 2016, the prevalence of IPIs was found to be 27.11%.
In this study population 71.18% were sputum smear positive. As per the data given in TB Statistics India 2016, in Odisha the incidence rate was 195 per 1 lac population, with a sputum positivity rate of 54% [5]. The high smear positivity rate in this study may be due to better facilities available and expertise in detection of AFB by ZN staining smear of sputum sample. This being a tertiary care hospital also receives a lot of referral cases from the eastern belt.
Due to a paucity of studies on co-infection rates in India, the results were compared with similar studies done in other countries.
In this study, the prevalence of IPIs among the study group was 27.11%. Findings of our study were comparable to studies from Ethiopia, in Arba Minch (26.3%) [10]. However, the prevalence was less as compared to another study done in Sub-Saharan Africa (40.3%) by Alemayehu M et al., [11]. This may possibly be due to the differences in the selection criteria of study participants; in our study the study population comprised of all newly diagnosed PTB patients, unlike the other study which also included known PTB patients taking anti-tubercular therapy. This observed difference may also be due to differences in study period, method of stool examination, geographical area and sample size.
In a study conducted in China, a relatively lower prevalence (14.9%) was seen in comparison to the current study [7]. This difference in the prevalence of IPIs in both the studies can be ascertained due to the differences in the economic status, the level of awareness about the intestinal parasite transmission and prevention approaches in the sampled population.
Amongst the study population, helminth co-infection was found to be the commonest (87.5%) and rest was due to protozoal infections (12.5%). Hookworm infection was found to be predominant i.e., 65.63% of the total parasite co-infected cases. When analysis of parasitic infection with respect to sputum smear status was done, among smear positives 22.6% of cases had hookworm infection where as 5.88% among smear negatives and this difference was also found to be statistically significant.
This result is in concordance with the findings of a study done in South India, and another study done in Egypt, by Hasanain AF et al., where Hookworm infection was found to affect 16.5% among the sampled study population with PTB; whereas it is lower than that reported by both Elias D et al., (28.3%) and Abate E et al., (25%) [12-15]. The outcome of TB infection depends on the cell-mediated immunity. Individual variation in susceptibility to TB is not fully understood. Susceptibility to TB is associated with reduced Th1 type responses and/or enhanced Th2 responses [16]. Responses of Th2 are usually elicited by helminth infections [16].
Prevalence of Strongyloides stercoralis in our study was found to be the second common parasitic infection i.e., 15.62%, with no significant association with sputum positive cases. Study by Resende Co T et al., [17] showed infection with S. stercoralis to be very high (i.e., 72.7%) of TB-helminthic co-infection. These differences may be because of the different geographical areas were the studies were done.
Ascaris lumbricoides was found to be the least common (6.2%) parasitic infection in our study with no significant association with sputum positivity status. In a study by Abate E et al., from north-west Ethiopia, Ascaris lumbricoides was the most common intestinal parasite species among helminth co-infections in patients with newly diagnosed TB [15].
The lower prevalence rate of Strongyloides stercoralis and Ascaris lumbricoides in our study cannot be explained. This can become clearer when further studies with larger sample size are done.
In this study, Entamoeba histolytica was found as the only protozoal infection and contributed 12.5% of the total parasitic co-infections. Association of E. histolytica infection was also found to be highly statistically significant with smear negative cases. Our study finding is higher than a study done among TB patients in China (1.4%) [7]. This might be due to variance in water supply, economic variation, feeding habit, environmental sanitation, sample size of study population and awareness of the means of transmission and prevention and control measure of this parasitic infection.
IPIs co-infection in our study was most (43.75%) among patients in age group 11-20 years; this was also statistically significant, followed by 21-30 years (18.75%). In a study by Li XX et al., intestinal parasite co-infections was more commonly seen in PTB patients >60 years age group [6]. In a study done in India to assess IPIs, it was found to be more prevalent among adults then children [1]. Our study findings on IPIs show a similar picture, and can be corroborated with the Indian study. IPIs being more prevalent in our country, previous parasitic infection before PTB infection can be been cited as the reason for such.
In our study co-infection was found more (62.5%) in male patients. Our finding is comparable with a South Indian study by Chatterjee S et al., which showed that males were at higher risk than females [18]. In contrast to our findings, a study from China [7] showed intestinal parasites being more common in females with PTB. This may be due to geographical differences in the place where the studies were done.
Limitation
As our study was an approved ICMR-STS project done over a time period of two months, larger sample size could not be recruited. A study with a larger sample size would make the results more generalizable. Owing to the cross-sectional nature of the study, a causality of association could not be made. Larger case-control study will give a better outcome.
Conclusion
The prevalence of intestinal parasites among newly diagnosed PTB patients attending the tertiary care hospital was 27.11%. The intestinal parasite co-infection amongst smear positive TB cases was 30.95%. The prevalence of helminthic infection especially hookworm was relatively higher among smear positive TB cases. As a known fact that PTB and IPIs are considered risk factors of each other and co-infection may inhibit the host immune system, therefore, health care providers should screen and treat TB patients for co-infection of intestinal parasites in order to ensure good prognosis and to set strategies for better case management. Further prospective studies should be carried out to confirm these findings.