Introduction
Periodontal disease comprises of a group of inflammatory diseases like periodontitis that affect the gingiva and supporting structures of the teeth [1]. It is well established in the past literature that around 5-15% of the population suffers from severe periodontitis. Gingivitis, a reversible periodontal disease may progress into periodontitis, an irreversible stage of the disease due to an imbalance between the resident microbiota and the host response [2]. While gingivitis affects only the gingival tissue, periodontitis often presents with bleeding on probing, increased pocket depth and clinical attachment loss.
It has been widely accepted that periodontitis is a result of the host immune inflammatory response caused by various periodontal microorganisms. This host response is mediated mainly by the immune cells that are triggered to produce various inflammatory mediators like cytokines, chemokines, arachidonic acid metabolites and proteolytic enzymes, which comprehensively lead to tissue degradation and II bone resorption [3]. One important mechanism of the inflammatory mediators present in periodontal tissue is the stimulation of the formation of osteoclasts that are believed to be the major cell type responsible for bone resorption [4].
The formation and the maintenance of the distinct membrane domains in osteoclasts during bone resorption is achieved by extensive membrane re-organisation and intracellular vesicle trafficking [5]. The major membrane trafficking pathways in osteoclasts have been resolved, but much is still enigmatic. The resorptive organ of osteoclasts, the ruffled border, is formed by the delivery of vesicles containing late endosomal and lysosomal enzymes to the bone-apposing plasma membrane. These enzymes are newly synthesised enzymes from the biosynthetic route or originate from the endo-cytotic pathway from the baso-lateral membrane domain. Soluble endo-cytotic markers and iron-loaded transferrin that are internalised at the baso-lateral membrane are accumulated at the ruffled border in resorbing osteoclasts. The baso-lateral membrane thus serves as a membrane reservoir for the lost membrane at the ruffled border during the transcytosis [6].
The noble therapies to alter the osteoclastic molecular activities of osteoclasts and their role in inflammatory diseases such as space infections activity in the bone resorption can aid in the prevention of the bone related disorders.
Certain bone diseases like osteoporosis, Pagets disease and rheumatoid arthritis, show increased osteoclast formation. Therefore, it is essential to study the signaling pathways regulating osteoclast differentiation in order to understand the skeletal system in pathological conditions. Osteoclast differentiation is stimulated by M-CSF and Receptor activator of nuclear factor kappa-B ligand (RANKL). M-CSF induces the proliferation and survival of osteoclast precursor cells through activation of ERK and Akt, whereas RANKL recruits TRAF6 to activate MAPKs, Akt, and NFATc1 to promote differentiation of osteoclast precursors to osteoclasts. In addition to RANKL signaling, costimulatory signaling provides robust NFATc1 induction through activation of calcium signaling [7].
Although significant signaling pathways for osteoclast differentiation have been unraveled, future studies of delicate regulatory networks involved in bone homeostasis are required for the development of useful therapeutic strategies. Notably, future studies should focus on scrutinising the exact mechanisms underlying RANKL-mediated activation of costimulatory signals for Receptor activator of nuclear factor κ B (RANK).
Bone resorption is necessary during bone growth, tooth eruption, healing of fracture and for the maintenance of blood calcium levels. In the process of bone resorption, the mineral and organic phases of the bone matrix are dissolved and degraded respectively. Ultimately, the balance between bone resorption and bone formation helps in maintaining bone volume and strength [8].
Physiology of Resorption
Throughout life, continuous physiological remodeling of bone is exclusively dependent on bone resorption. In order to maintain stability and integrity of bone, it constantly undergoes remodeling, with about 10% of bone material being renewed each year. The process of bone remodeling involves bone resorption by osteoclasts and bone formation by osteoblasts. This complex process in both physiologic and pathologic instances is modulated by enzymes, hormones and the RANK/RANKL/OPG (Osteoprotegerin) system.
Osteoclasts are multinucleated giant cells that express genes whose activities are critical for resorption. The osteoclast is a polykaryon that normally contains up to eight nuclei. Its in-vitro diameter can reach 300 mm that enables the osteoclast to cover a relatively large matrix area and thereby operate efficiently. It acquires a polarised morphology in order to resorb bone [9].
The process of resorption occurs in the following steps [10]:
Fusion of the mononuclear precursors to form the polykaryon and target the site of resorption
Attachment to the mineralized bone matrix, reorganisation of actin and formation of the “actin ring” and the sealing zone.
Formation of the ruffled border, encircled by the sealing zone.
Release of acid and acidic collagenolytic enzymes into the space enclosed by the matrix, sealing zone and ruffled border resulting in mineral dissolution and organic matrix degradation.
Removal of the resorption products from the resorption lacuna to the functional secretary domain by transcytosis and their secretion into the circulation.
Migration and Targeting
Type I collage peptides, alpha-2-HS glycoprotein and osteocalcin are known to elicit a dose-dependent chemotactic response in human monocytes. The osteoclast precursors namely monocytes are mobilised by chemotaxis. Certain chemo-attractants that draw the resorptive cell to the remodeling site are derived either from the bone matrix or directly from the osteoblasts [11]. Another chemo-attractant responsible for this activity is Stromal cell-derived factor-1 (SDF-1), which is produced by immature osteoblasts within bone.
Osteoclasts: Culprits in Inflammatory Osteolysis
The osteoclast precursor arises mainly in the marrow as an early mononuclear macrophage [Table/Fig-1]. It then circulates and binds to the bone surface, followed by which, it fuses with its sister cells to form a polykaryon that does not replicate further [12]. Upon bone contact, the osteoclast polarises and possesses the capacity to degrade organic and inorganic components of the skeleton. Evidence suggests that the in-vivo life span of osteoclast is about 2 weeks.
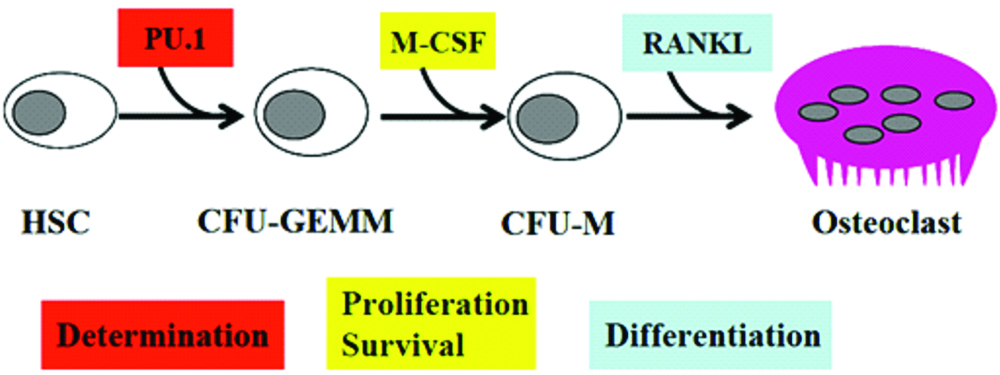
Osteoblast and Osteoclast Interaction
Osteoclastic Bone Resorption
Adhesion of osteoclasts to the bone surface involves integrins like αvβ3 with extracellular matrix proteins within the bone matrix. The surface receptors expressed by osteoclasts allows the osteoclast to attach to the surface by binding to certain adhesion ligands such as vitronectin that are present on the bone surface [13].
Osteoclasts Attach to Bone Matrix through the Sealing Zone: (Cell Biology of Osteoclast)
Intergrins are known to play an important role in the early phases of resorption. The four integrins expressed in osteoclasts are αvβ3, αβ5, α2β1 and αβ1, out of which, αvβ3 has gained attention because it interacts with arginine, glycine, aspartic acid (RGD) containing peptides [14]. Evidence indicates that αvβ3 could play a role in adhesion and migration of osteoclasts, in endocytosis of resorption products and also in mediating the attachment of sealing zone to the bone matrix. On the other hand, the RGD-containing peptides have the ability to inhibit osteoclastic bone resorption in vitro, by binding to the integrin receptor [Table/Fig-2].
Mechanism of osteoclastic bone resorption
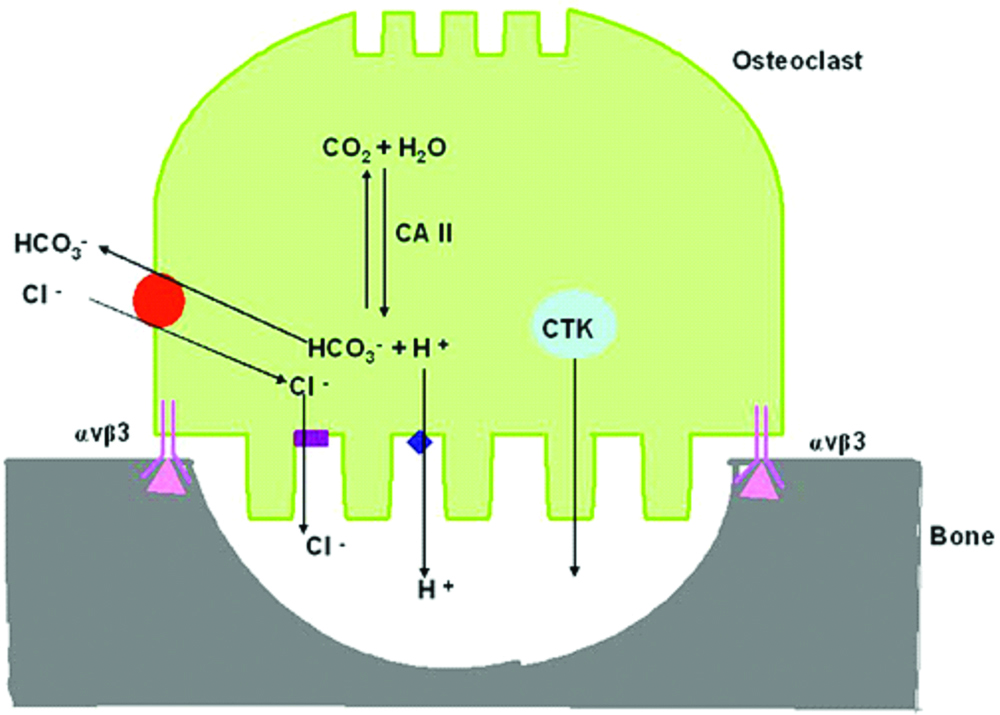
After the osteoclast gets attached to the bone surface, the process of polarisation allows the osteoclasts to produce 3 different membrane domains out of which the first is the sealing zone.
First Membrane Domain: The Sealing Zone
The sealing zone is a distinct compartment in osteoclasts that consists of a large amount of filamentous actin (F-actin). The sealing zone encircles the plasma membrane area where the ruffled border will be formed. The tight cell-to-matrix interaction “seals” the bone surface from the external environment and this tight seal, along with the F-actin, forms a ring-like structure around the resorption compartment [15]. The F-actin in the sealing zone localises in plasma membrane protrusions called podosomes. These podosomal structures reveal a central actin core that is surrounded by several actin-binding proteins such as paxillin, talin and vinculin-containing rosettes [Table/Fig-3]. The proteins that regulate the core and ring structure have the potential to regulate podosome turnover. However, it should be noted that this general pattern of the F-actin ring disappears on the formation of the final actin ring.

Podosomes and Their Proteins
One category of proteins present in the podosomes contain an F-actin cylindrical core comprising of actin regulatory proteins such as actinin, gelsolin (GSN), Wiskott-Aldrich syndrome protein (WASP), Arp2/3, cortactin and fimbrin. Another category of proteins that surround the core are adhesion molecules like integrins, adaptors (Cbl, kinases, Pyk2, etc.,) small GTPases (Rho and Rac) and endocytic regulators (dynamin, endophilin 2, etc.,) [16].
Integrin αvβ3
The integrin αvβ3 plays a major role in matrix recognition, organisation of osteoclast cytoskeleton and osteoclastic bone resorption. It also mediates osteoclastic motility and enhances osteoclastogenesis. Integrins which act as cell-cell and cell-matrix adhesion receptors, are activated by two basic mechanisms: i) the outside-in activation is by ligand-induced conformational change in the intergrin; ii) the inside-out activation is by the occupation of the receptor with a cytokine or growth factor which target the integrin’s cytoplasmic domain [17].
Src
The functions of Proto-oncogene tyrosine-protein kinase Src (Src) include secretion, adhesion to the extracellular matrix, motility, cell cycle control, proliferation and differentiation. Src is mainly concentrated in the actin ring and at the periphery of osteoclasts where attachment and migration takes place. Src has a remarkable role in osteoclast physiology. It also acts an adaptor protein and a tyrosine kinase, both the activities of which are required for resorptive activity [17].
Pyk2
Pyk2 is a member of the Focal adhesion kinase (FAK) family and is an important regulator of osteoclast function. Pyk2 is also involved in tyrosine kinase activity and is recruited to podosome adhesion structures in osteoclasts. Inhibition of Pyk2 expression leads to a decrease in bone resorption suggesting that it plays an important role in cytoskeletal reorganisation [18].
Cbl
Casitas B-lineage lymphoma (Cbl) is an adaptor protein and is the product of a proto-oncogene. It has been reported to be a Src-dependent substrate in osteoclasts, which is required for bone resorption. Cbl organises associated proteins like Src into larger signaling complexes such as the Cbl-Src-Pyk2 tri-molecular signaling complex in osteoclasts. This signaling complex has been found to be important for osteoclast adhesion, migration and resorption [19].
Dynamin
Dynamin is a GTP-hydrolyzing protein that is involved in actin remodeling in podosomes and thereby possibly in cell migration and bone resorption. Dynamin binds to a number of SH3-containing molecules like Src, cortactin, amphiphysin, Growth factor receptor-bound protein 2 (Grb2) and endophilin. Dynamin in association with Cbl via Grb2 enhances GTPase activity and WASP-mediated Arp2/3-dependent actin nucleation. Dynamin also interacts with Pyk2 and leads to decreased Src binding to Pyk2. Therefore it can be stated that dynamin may provide a Src-dependent “off-switch” that dissembles that Pyk2-Src-Cbl complex and down regulates its downstream signaling [20].
Cortactins
Cortactin is a substrate of Src. Reduction of coractin in the osteoclasts by RNA which does not form sealing zones. It also causes a complete loss of podosomes. This is incapable of bone resorption. In addition, coractin is thought to stabilise actin filament branches formed by the Arp2/3 complex and activate this complex that is essential for actin nucleation [21].
Guanine Nucleotide Exchange Proteins
Rearrangement of actin structures is mediated by a family of proteins called the Rho family of small guanine nucleotide exchange proteins. These Rho family of GTPases (Rho-GTPases) are known to promote F-actin adhesion structure rearrangements and microtubule destabilisation thereby mediating cross-talk between the two. Once the osteoclasts get attached to the bone, Rho is activated in a β3-integrin dependent manner and translocates to the cytoskeleton. The guanine nucleotide exchange proteins also convert the Rho-GTPase from the inactive GDP bound state to their active GTP bound state [22,23].
Rab Family and Vascular Trafficking
Rab family are essential components of cell and organelle membranes and regulate vesicular trafficking during endocytosis, exocytosis, and other vesicular changes. As the vesicular transport is critical in OCs not only for autophagy but also for the essential bone resorption functions, the mammalian Rab GTPases plays a role in the regulation of vesicle trafficking during autophagy. The associations of Rab proteins with bone resorbing functions of OCs have also been noticed. Rabs also interact with numerous sorting adaptors, tethering factors, kinases, and phosphatases. Active Rabs predominantly consolidate and moderate intracellular trafficking signals by enlisting various effectors to define distinct microdomains on membrane surface for regulatory molecules and downstream effectors [24].
Second and Third Membrane Domains
The polarisation of the sealing zone helps in the formation of 2 more membrane domains namely the baso-lateral domain and the ruffled border. The baso-lateral domain is basically the cellular membrane that is exposed to the external environment and is the body of the osteoclasts. Another region that has been identified to be a part of the baso-lateral membrane is the functional secretory domain.
The Ruffled Border and Its Formation
The ruffled border is considered to be the most functionally important membrane domain and the resorbing organelle of the osteoclasts. It has not been identified in any other membrane of other cells. This membrane comprises of complex infoldings formed via the fusion and subsequent insertion of intracellular acidic vesicles from the osteoclast plasma membrane. This fusion process reorganises the plasma membrane to form long, finger like projections that penetrate the bone matrix forming the resorption lacunae that are considered to be the resorbing component of osteoclasts. The ruffled border highly expresses V-type ATPases that have provided researchers pathways to control bone diseases by using specific inhibitors that bind to the ATPase subunits thereby controlling bone resorption in-vitro [25].
During the early phase of resorption, the ruffled border is formed by fusion of intracellular acidic compartments to the bone-facing plasma membrane. This fusion releases hydrochloric acid and proteolytic enzymes like cathepsin K into the resorption site. Once polarised, the process of bone degradation begins. Thus, there must be a membrane cycling mechanism at the level of the ruffled border that allows the simultaneous secretion of acid and proteolytic enzymes from the cell and, on the other hand, the uptake of bone degradation products into the cell.
Osteoclasts and Periodontitis
Osteoclasts are responsible for bone resorption during normal bone remodeling and inflammatory diseases like periodontitis. Periodontal diseases mainly periodontitis result from a complex interplay between the subgingival biofilm and the host immune inflammatory response that develop in the periodontal tissues in response to bacteria [26].
Inflammatory Mediators and Bone Resorption
Immune cells and bone cells both originate from bone marrow environment and are closely functionally related. Crosstalk between the bone and the immune system is important for bone homeostasis and physiological bone remodelling [27]. Members of the Tumour necrosis factor (TNF)-related family of ligands and receptors, as well as other cytokines, colony stimulating factors, and signalling molecules are essential for normal development and function of both systems. This has been well documented in many experimental models and human diseases [28]. Moreover, it has been shown that bone and immune cells may share the same progenitors and that their differentiation may be driven by the same support cells. Multiple complex interactions between hematopoietic and mesenchymal lineage cells define the bone marrow micro-environment.
The most important inflammatory mediators that stimulate osteoclast activation are cytokines such as Interleukin-1-beta (IL-1β), tumor necrosis factor alpha (TNF-α), Interleukin-6 (IL-6), Macrophage colony-stimulating factor (M-CSF), 7-Interleukin-17 (IL-17) and Prostaglandin-E2 (PGE2) [29].
RANKL, a TNF family cytokine, is known to induce the differentiation of osteoclasts in the presence of M-CSF. It also activates TRAF6 (membrane of TNF receptor associated factor), c-Fos and calcium signaling pathways, which are essential for the induction and activation of Nuclear factor of activated T cells (NFAT), a key transcription factor for osteoclastogenesis. Wingless/Integrated (Wnt5a), a member of the Wnt protein family, is regarded as a new costimulatory cytokine for osteoclastogenesis by upregulating RANK expression [Table/Fig-4].
M-CSF/RANKL signaling pathways.
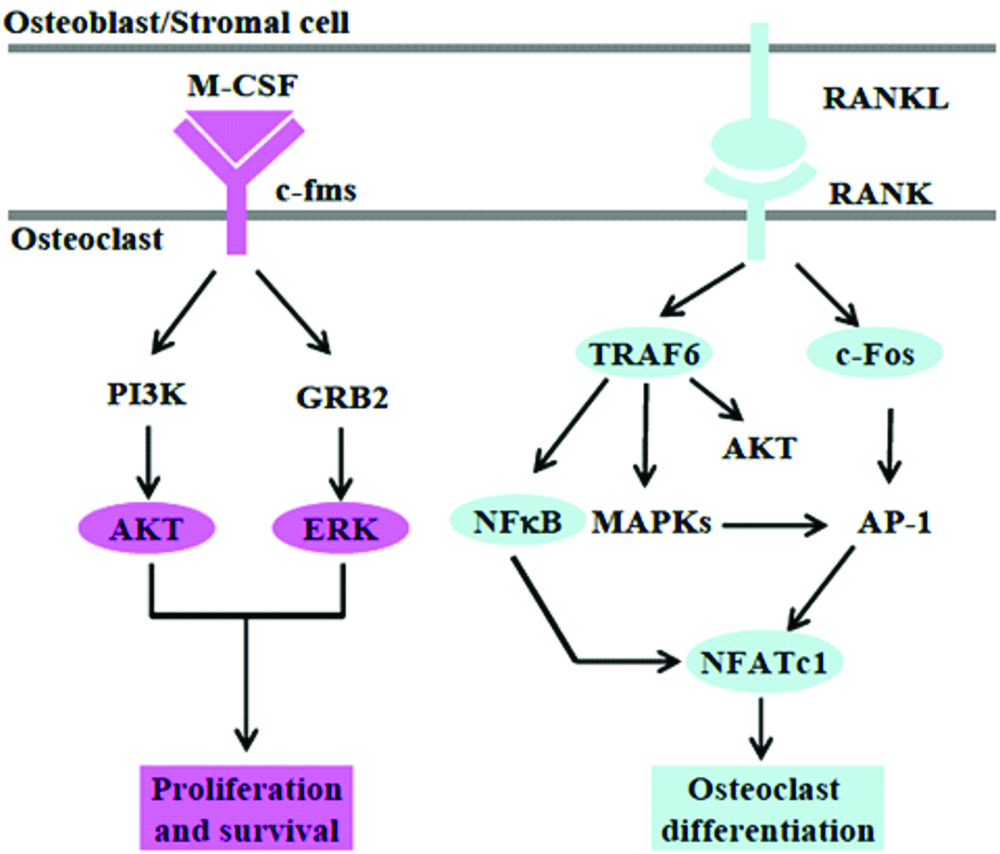
OPG, a decoy receptor for RANKL prevents RANKL from binding to its receptor RANK. Thus, inhibition of OPG enables RANKL to interact with RANK thereby causing differentiation of osteoclasts. The RANKL/OPG ratio was higher in gingival crevicular fluid samples of patients with periodontitis suggesting that increased RANKL and/or decreased OPG contribute to bone resorption.
Furthermore, IL-1 and TNF also increase osteoclast formation thereby stimulating bone resorption. IL-1β induces RANKL and/or OPG expression in various cells such as gingival fibroblasts, osteoblasts and periodontal ligament fibroblasts. Similarly, IL-6 secreted by various cells stimulates bone resorption.
PGE2 stimulates bone resorption via the up-regulation of RANKL and inhibition of OPG in osteoblasts. Thus, PGE2 and lipopolysaccharides can intensify this process of osteoclastogenesis through direct effects on the haematopoietic cell lineage.
Both microbial and host factors play a role in tissue destruction leading to periodontitis which is the result of a host inflammatory reaction to the local accumulation of microorganisms in the proximity of dento-gingival junction or by direct assault of microorganisms and their products on periodontal tissues. The initial stages of periodontitis involve leukocyte infiltration and migration, alteration of fibroblasts, loss of collagen fibers and proliferation of the cells in the junctional epithelium. In the advanced stages of periodontitis, the periodontal ligament and alveolar bone are resorbed by activated osteoclasts.
Action of the Bisphosphonates on Osteoclasts
The bisphosphonates are considered safe and efficacious. They are usually in close contact with osteoclasts due to their selective uptake that has been illustrated using various radiolabeling techniques. Bisphosphonates can affect osteoclast function in various ways, including osteoclast recruitment, differentiation, resorptive activity and some may cause apoptosis of osteoclasts.
Concluding Remarks
The osteoclast is one of the many elements involved in a highly convoluted osteoclastic system. Periodontal disease sites provide the proper environment for the development of many of the agents that attract and activate elements of the osteoclastic system. Microbial agents, inflammatory cells and their products contribute to a complex pattern in which the alveolar bone is lost to osteoclast activity. Many ways seem open to alveolar destruction and depending on the microbial flora and host response, one or more of these pathways may produce various rates of active osteoclastic bone resorption.
[1]. Kim J, Amar S, Periodontal disease and systemic conditions: Bidirectional relationshipOdontology 2006 94:10-21.10.1007/s10266-006-0060-616998613 [Google Scholar] [CrossRef] [PubMed]
[2]. Offenbacher S, Periodontal diseases: pathogenesisAnn. Periodontal 1996 1(1):821-78.10.1902/annals.1996.1.1.8219118282 [Google Scholar] [CrossRef] [PubMed]
[3]. Page RC, The role of inflammatory mediators in the pathogenesis of periodontal diseaseJ Periodontal Res 1991 26(3):230-42.10.1111/j.1600-0765.1991.tb01649.x1679130 [Google Scholar] [CrossRef] [PubMed]
[4]. Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernández M, Gamonal J, Host response mechanisms in periodontal diseasesJ Appl Oral Sci 2015 23:329-55.10.1590/1678-77572014025926221929 [Google Scholar] [CrossRef] [PubMed]
[5]. Väänänen HK, Zhao H, Mulari M, Halleen JM, The cell biology of osteoclast functionJ Cell Sci 2000 113:377-81. [Google Scholar]
[6]. Stenbeck G, Formation and function of the ruffled border in osteoclastsSemin Cell Dev Biol 2002 13(4):285-92.10.1016/S1084952102000587 [Google Scholar] [CrossRef]
[7]. Jung Ha Kim, Nacksung Kim, Signaling Pathways in Osteoclast DifferentiationChonnam Med J 2016 52(1):12-17.10.4068/cmj.2016.52.1.1226865996 [Google Scholar] [CrossRef] [PubMed]
[8]. Prashant Babaji, Raghu Devanna, Kiran Jagtap, Vishwajit Rampratap Chaurasia, Jeethu John Jerry, Basanta Kumar Choudhury, Dinesh Duhan, The Cell Biology and Role of Resorptive Cells in Diseases: A ReviewAnn Afr Med 2017 16(2):39-45.10.4103/aam.aam_97_1628469115 [Google Scholar] [CrossRef] [PubMed]
[9]. Ikeda K, Takeshita The role of osteoclast differentiation and function in skeletal homeostasisJ Biochem 2016 159:01-08.10.1093/jb/mvv11226538571 [Google Scholar] [CrossRef] [PubMed]
[10]. Bar-Shavit Z, The osteoclast: a multinucleated, hematopoietic-origin, bone-resorbing osteoimmune cellCell Biochem 2007 102:1130-39.10.1002/jcb.2155317955494 [Google Scholar] [CrossRef] [PubMed]
[11]. Malone JD, Teitelbaum SL, Griffin GL, Senior RM, Kahn AJ, Recruitment of osteoclast precursors by purified bone matrix constituentsJ Cell Biol 1982 92:227-30.10.1083/jcb.92.1.2276976967 [Google Scholar] [CrossRef] [PubMed]
[12]. Teitalbum SL, Bone resorption by osteoclastsScience 2000 289:1504-1508.10.1126/science.289.5484.150410968780 [Google Scholar] [CrossRef] [PubMed]
[13]. Phan TC, Xu J, Zheng MH, Interaction between osteoblast and osteoclast: impact in bone diseaseHistol Histopathol 2004 19:1325-44. [Google Scholar]
[14]. Nesbitt S, Helfrishm A, Horton M, Biochemical characterization of human osteoclast integrin’s. Osteoclast expresses αvβ3, α2β1 and αvβ1 integrinsJ biol chem 1993 268:16737-45. [Google Scholar]
[15]. Väänänen HK, Horton M, The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structureJ Cell Sci 1995 108:2729-32. [Google Scholar]
[16]. David JM, Cecile I, Miep HH, The skeleton: a multifunctional complex organ. The role of key signalling pathways in osteoclast differentiation and in bone resorptionJ Endocrinol 2011 211:131-43.10.1530/JOE-11-021221903860 [Google Scholar] [CrossRef] [PubMed]
[17]. Bruzzaniti A, Baron R, Molecular regulation of osteoclast activityRev Endocr Metab Disord 2006 7:123-39.10.1007/s11154-006-9009-x16951988 [Google Scholar] [CrossRef] [PubMed]
[18]. Soysa NS, Alles N, Osteoclast function and bone-resorbing activity: An overviewBiochem Biophys Res Commun 2016 29:115-20.10.1016/j.bbrc.2016.05.01927157135 [Google Scholar] [CrossRef] [PubMed]
[19]. Lakkakorpi PT, Nakamura I, Nagy RM, Parsons JT, Rodan GA, Duong LT, Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zoneJ Biol Chem 1999 274(8):4900-07.10.1074/jbc.274.8.49009988732 [Google Scholar] [CrossRef] [PubMed]
[20]. Bruzzaniti A, Neff L, Sandoval A, Du L, Horne WC, Baron R, Dynamin reduces Pyk2 Y402 phosphorylation and SRC binding in osteoclastsMol. Cell. Biol 2009 29(13):3644-56.10.1128/MCB.00851-0819380485 [Google Scholar] [CrossRef] [PubMed]
[21]. Tehrani S, Faccio R, Chandrasekar I, Ross FP, Cooper JA, Cortactin has an essential and specific role in osteoclast actin assemblyMol. Biol. Cell 2006 17(7):2882-95.10.1091/mbc.e06-03-018716611741 [Google Scholar] [CrossRef] [PubMed]
[22]. Rojas AM, Fuentes G, Rausell A, Valencia A, Te Ras protein superfamily: Evolutionary tree and role of conserved amino acidsTe Journal of Cell Biology 2012 196(2):189-201.10.1083/jcb.20110300822270915 [Google Scholar] [CrossRef] [PubMed]
[23]. Roy M, Roux S, Rab GTPases in Osteoclastic Endomembrane SystemsBioMed Research International 2018 :01-15.10.1155/2018/454153830186859 [Google Scholar] [CrossRef] [PubMed]
[24]. Baron R, Neff L, Roy C, Boisvert A, Caplan M, Evidence for a high and specific concentration of (Na+,K+) ATPase in the plasma membrane of the osteoclastCell 1986 18:311-20.10.1016/0092-8674(86)90748-8 [Google Scholar] [CrossRef]
[25]. Julie C Crockett, Michael J Rogers, Fraser P Coxon, Lynne J Hocking, Miep H Helfrich, Bone remodelling at a glanceJournal of Cell Science 2011 124:991-98.10.1242/jcs.06303221402872 [Google Scholar] [CrossRef] [PubMed]
[26]. Xu F, Steven L, Teitelbaum. Osteoclasts: New InsightsBone Res 2013 Mar 1(1):11-26.10.4248/BR20130100326273491 [Google Scholar] [CrossRef] [PubMed]
[27]. Hienz SA, Paliwal S, Ivanovski S, Mechanisms of Bone Resorption in PeriodontitisJ Immunol Res 2015 2015:61548610.1155/2015/61548626065002 [Google Scholar] [CrossRef] [PubMed]
[28]. Mori G, D’Amelio P, Faccio R, Brunetti G, The Interplay between the Bone and the Immune SystemClin Dev Immunol 2013 2013:72050410.1155/2013/72050423935650 [Google Scholar] [CrossRef] [PubMed]
[29]. Bhanu MM, Osteoimmunology-Unleashing the conceptsJ Indian Soc Periodontol 2011 15:190-98.10.4103/0972-124X.8565922028503 [Google Scholar] [CrossRef] [PubMed]