Diamond blackfan anaemia (DBA) is an inherited bone marrow failure syndrome characterised by severe anaemia resulting in depletion or absence of erthyroid progenitor cells and haemopoesis. The pathophysiology of DBA is still undescribed. It is usually manifested in infancy but is not restricted to paediatric patients. Sometimes it might predispose to malignancy. In this paper, we present a single case of DBA with clear oral findings. This case report describes the medical importance of this condition and its risk in dental management (tooth extraction under local anaesthesia).
Diamond-blackfan type, Extraction, Hypodontia, Tooth
Case Report
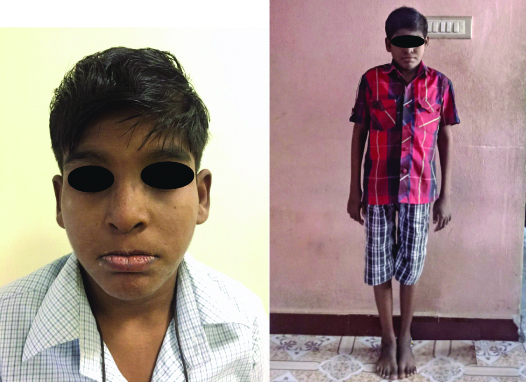
A 21-year-old male patient presented to the Department of Oral Medicine and Radiology with the chief complaint of missing teeth and wanted the replacement of his teeth. The patient’s medical history revealed chronic anaemia since childhood and was diagnosed with DBA at the age of four and Status Epilepticus at age of 11. He was advised the following medication (Tab. Phenytoin, Tab. Levetiracetam, Tab. Carbamazepine, Tab. Clonazepam). Patient’s personal and family history revealed that he is the first child and parents had a consanguineous marriage; the other sibling of the patient was apparently healthy and normal. The Physical examination of the patient revealed short stature (141 cm), weighing 21 kg only [Table/Fig-1], which is a typical sign in the case of DBA and also presented with skeletal abnormalities such as short shoulder blade, dryness of skin, and visual disturbances. The patient also had learning difficulties. The patient was transfused blood every week to maintain the Haemoglobin concentration of around 10 gm%. The patient was under corticosteroids for a period of two years, but it was later found that the patient was not responding to steroidal therapy; thus the steroidal therapy was discontinued.
Image of the patient showing short stature, short shoulder blade and dryness of the skin.
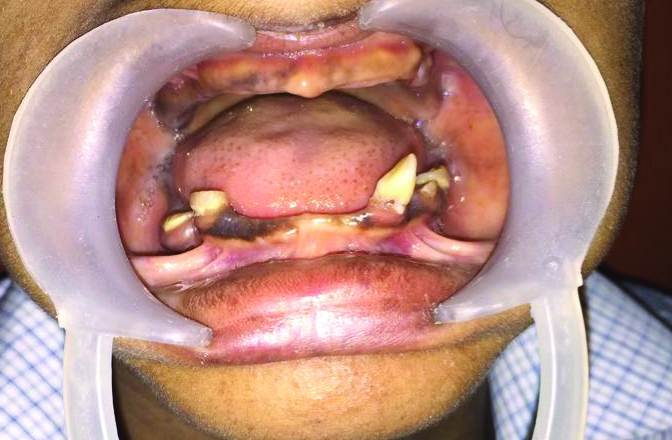
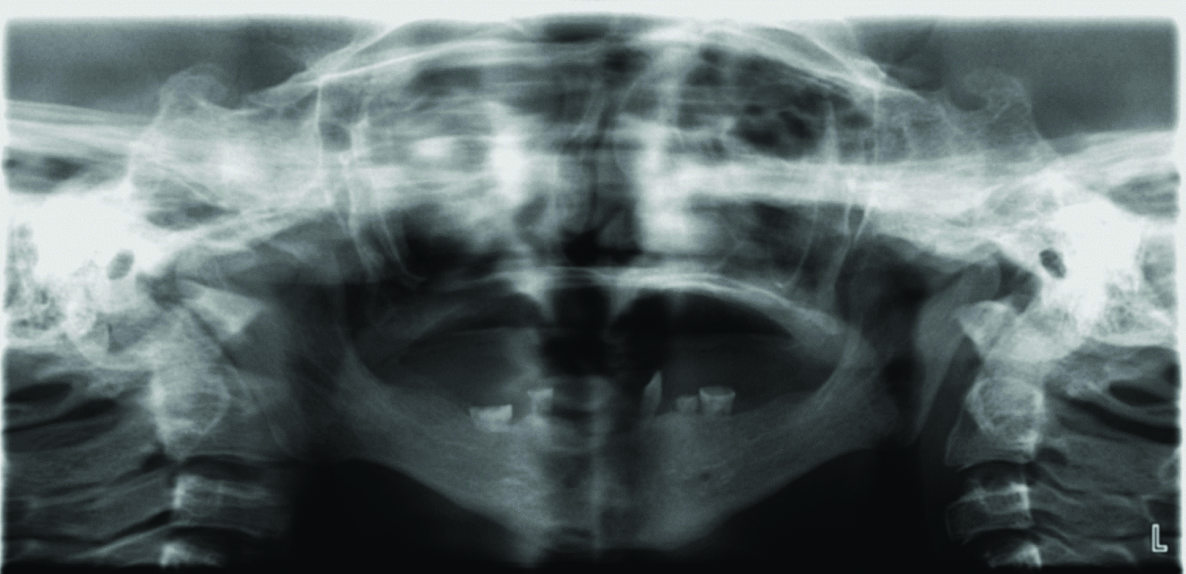
Intraoral examination revealed complete absence of teeth in the upper jaw and presence of few teeth in the lower jaw. The deciduous teeth present were 73,74,75,83 and 84. The maxillary and mandibular bone appeared to be atrophic [Table/Fig-2]. Orthopantomograph revealed the complete absence of primary and permanent tooth buds in maxilla and presence of five primary teeth in the mandible and the absence of permanent tooth buds. The root resorption was evident in mandibular right and left first and second molar [Table/Fig-3].
Preoperative orthopantomogram.
The partial absence of deciduous teeth and complete absence of permanent teeth was confirmed with clinical and radiological findings. Patient was planned for total extraction under local anaesthesia. On the day of the dental procedure, hemoglobin concentration of the patient was 9.7g/dL; mean corpuscular volume was 86 fL, the platelet count (1.55 Lac cu/mm) and white blood cell counts were within normal range (5400 cells/cumm). Bleeding time was 2 minutes and Clotting time was 4 minutes. Medical fitness was obtained from the Department of Neuromedicine and the Department of haematology for the procedure. Neuromedicine department advised that patient can be taken up for the extraction of tooth, but with a risk of periprocedural seizure. Patient attendant gave consent for tooth extraction.
The patient was then referred to Department of Oral and Maxillofacial Surgery for extraction of remaining teeth for prosthetic rehabilitation.
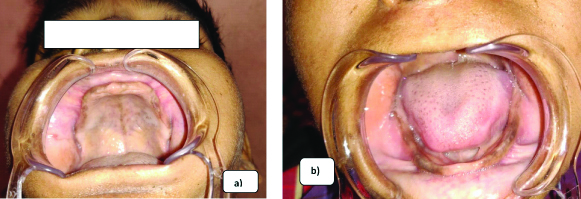
Under aseptic conditions, extraoral and intraoral painting was done with 10% betadine solution and cardiac monitoring was done (Sp O2, Hr. RR). A 2% xylocaine with adrenaline was administered and bilateral inferior alveolar nerve block was given. Uneventful extraction of the teeth was performed. Hemostasis was achieved. Postoperatively there were no complications. Patient was given oral antibiotics and analgesics and was reviewed after a month [Table/Fig-4]. Healing was satisfactory. At present, the patient is being followed up for a period of 3 months. The patient has been referred for full mouth rehabilitation.
Postoperative Intraoral Photograph: a) Maxillary arch; b) Mandibular arch.
Discussion
DBA is a chronic pure red cell aplasia characterised by congenital anomalies such as anaemia, growth retardation and microcephaly [1]. This condition occurs early in infancy, which is not restricted to paediatric patients [2]. The typical symptom of DBA is anaemia, which is present from birth or within first year of life [3]. Physical abnormalities such as microcephaly, cleft lip and palate, reduction in height, webbed neck and decreased shoulder blade, defects in upper limb, as well as the eyes, heart, urinary tract, kidney and liver are manifested [4]. There is no sex predilection for DBA. DBA shows autosomal dominant or a recessive pattern of inheritance in 10-20% of patients [5,6]. Low birth weight and growth retardation has been reported in literature with no specific dental finding [4]. The first mutated gene in DBA is ribosomal protein S19 (RP S19) [7].
The haematological characteristics of DBA are normocellular bone marrow with deficiency of red blood cell precursors, normochromic macrocytic anaemia, reticulocytopenia, normal and slightly reduced leukocyte counts and normal or decreased platelet count [1,4,8].
Sometimes DBA is associated with malignancy often with Acute myeloid leukemia (AML) [4]. Treatment approach includes, corticosteroids, blood transfusion, bone marrow transplantation interleukin therapy, and iron chelating therapy [1,8,9]. Most of the patients give a positive response to corticosteroids [3]. Other treatment modalities for DBA include chronic red cell infusion combined with iron chelating therapy or allogenic Bone marrow transplantation (BMT) from HLA-identical siblings. The condition being a lifetime disorder requires a longtime follow-up [10,11].
This anaemia was first quoted in the year 1936 by HW Josephs and later by LK Diamond and KD Blackfan [11,12]. Prevalence of the disease is one in every 1,00,000 to 2,00,000 inhabitants, with no ethnicity and gender predisposition [7]. Congenital hypoplastic anaemia is characterised by macrocytic anaemia, neutropenia, thrombocytosis, and deficiency of erythroblast in bone marrow [13,14].
The disease is successfully treated with corticosteroid therapy; however, patients who do not respond to corticosteroids are followed-up with transfusion. Chronic blood transfusion causes iron overload. The same issue occurred in our patient due to the chronic blood transfusion, which increased the iron content in various parts of the body. At present, the patient is on iron chelating therapy. Other than corticosteroids and blood transfusion, Stem cell transplantation (SCT) can be performed. SCT includes bone marrow, peripheral blood stem, and cord blood. A previous study concluded that in children with DBA, short stature and growth retardation, the growth hormone therapy should be considered [15]. The underlying disease condition and hereditary characteristics are important for genetic counseling.
In 2000, Fredman MH categorised the phenotypic abnormalities according to: (a) craniofacial dysmorphism, microcephaly, congenital cataracts or glaucoma; (b) prenatal or post-natal growth failure; (c) neck abnormalities; (d) thumb malformation, craniofacial deformities, microcephaly and high-arch palate [3]. Post-natal growth failure is also seen, but in our patient, no such abnormalities were detected.
Later in 2001, Gripp KW et al., stated midfacial hypoplasia, cleft palate, downslanting palpebral fissure, bilateral microtia, and micrognathia in two cousins with DBA, but in our case patient’s sibling did not suffer from any of the following and no such abnormalities were found in our patient [16]. None of the reported cases of DBA had status epileptics and congenitally missing teeth, which was present in our patient. The previous literature also cites that tooth extraction causes delayed wound healing [17].
Reports in the literature describing oral and dental findings have not included details regarding extraction of the tooth. Therefore, our paper is the first to report extraction of teeth without any post-extraction complications and satisfactory healing. Based on the clinical condition, whether status epilepticus could be a part of this syndrome needs to be investigated.
Conclusion
In our case, we observed that there was partial absence of deciduous and complete absence of permanent teeth. No such dental anomalies have been reported in literature in DBA. Therefore, this case is the first to report dental anomaly in the patient of DBA with satisfactory healing on extraction.
[1]. Da Costa L, Willig TN, Fixler J, Mohandas N, Tchernia G, Diamond-Blackfan AnemiaCurr Opin Paediatr 2001 13(1):10-15.10.1097/00008480-200102000-00002 [Google Scholar] [CrossRef]
[2]. Alter BP, Diagnosis, genetics, and management of inherited bone marrow failure syndromesHaematology Am Soc Hematol Educ Program 2007 :29-39.10.1182/asheducation-2007.1.2918024606 [Google Scholar] [CrossRef] [PubMed]
[3]. Freedman MH, Diamond-blackfan anemiaBillire’s Clinical Hematology 2000 13:391-406.10.1053/beha.2000.008411030041 [Google Scholar] [CrossRef] [PubMed]
[4]. Vlachos A, Klein GW, Lipton JM, The Diamond Blackfan anemia registry: Tool for investigating the epidemiology and biology of Diamond-Blackfan anemiaJ Paediatr Hematol Oncol 2001 23:377-82.10.1097/00043426-200108000-0001511563775 [Google Scholar] [CrossRef] [PubMed]
[5]. Young NS, Aplastic anemia: Acquired and Inherited 1994 PhiladelphiaWB Saunders Co:361-83. [Google Scholar]
[6]. Alter BP, The bone marrow failure syndrome. In: Nathan DG, Orkin SH (eds)Nathan and Oski’s. Hematology of infancy and childhood 1998 PhiladephiaWB Saunder Co:237-335. [Google Scholar]
[7]. Flygare J, Aspesi A, Bailey JC, Miyake K, Caffrey JM, Karlsson S, Human RPS19, the gene mutated in Diamond-Blackfan anemia, encodes a ribosomal protein required for the mutation of 40S ribosomal subunitsBlood 2007 109(3):980-86.10.1182/blood-2006-07-03823216990592 [Google Scholar] [CrossRef] [PubMed]
[8]. Krijanovski OI, Sieff CA, Diamond-Blackfan anemiaHematol Oncol Clin North Am 1997 11:1061-77.10.1016/S0889-8588(05)70483-4 [Google Scholar] [CrossRef]
[9]. Vlachos A, Ball S, Dahl N, Alter BP, Sheth S, Ramenghi U, Participants of sixth Annual Daniella Maria Arturi International Consensus Conference. Diagnosing and treating Diamond-Blackfan Anemia: results of an international clinical consensus conferenceBr J Haematol 2008 142:859-76.10.1111/j.1365-2141.2008.07269.x18671700 [Google Scholar] [CrossRef] [PubMed]
[10]. Mushigima H, Gale RP, Rowling PA, Horowitz MM, Marmont AM, McCann SR, Bone marrow transplantation for Diamond-Blackfan anemiaBone Marrow Transplant 1995 15:55-58. [Google Scholar]
[11]. Joseph HW, Anemia of infancy and early childhoodMedicine 1936 15:30710.1097/00005792-193615030-00001 [Google Scholar] [CrossRef]
[12]. Diamond KL, Blackfan KD, Hypoplastic anemiaAm J Dis Child 1938 56:46410.1001/archpedi.1938.01980140227015 [Google Scholar] [CrossRef]
[13]. Halperin DS, Freedman MH, Diamond-Blackfan anemia: etiology, pathophysiology, and treatmentAm J Paediatr Hematol Oncol 1989 11:380-94. [Google Scholar]
[14]. Gazda HT, Sieff CA, Recent insights into the pathogenesis of Diamond-Blackfan anemiaBr J Haematol 2006 135:149-57.10.1111/j.1365-2141.2006.06268.x16942586 [Google Scholar] [CrossRef] [PubMed]
[15]. Scott EG, Haider A, Hord J, Growth Hormone Therapy for Short Stature in Diamond Blackfan AnemiaPaediatr Blood Cancer 2004 43(5):542-44.10.1002/pbc.2007515382270 [Google Scholar] [CrossRef] [PubMed]
[16]. Gripp KW, McDonald-McGinn DM, Rossa DL, McGain D, Federman N, Vlachos A, Bilateral microtia and cleft palate in cousins with Diamond-Blackfan anemiaAm J Med Genet 2001 101:268-74.10.1002/ajmg.1329 [Google Scholar] [CrossRef]
[17]. Sanger RG, Wilson GA, Congenital hypoplastic anemia (Diamond-Blackfan syndrome)J Oral Med 1982 37:08-13. [Google Scholar]