Clinico-mycological Study of Otomycosis Comparing the Cavity Slide Technique and the Conventional Agar Block Slide Culture
Tabindah Jahan1, Mohsin Bin Mushtaq2, Nargis Bali3, Sabah Nargis4, Yousuf Ul Bashir5
1 Senior Resident, Department of Microbiology, Government Medical College, Srinagar, Jammu and Kashmir, India.
2 Postgraduate, Department of Medicine, Government Medical College, Srinagar, Jammu and Kashmir, India.
3 Assistant Professor, Department of Microbiology, Sher e Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India.
4 Senior Resident, Department of Physiology, Government Medical College, Srinagar, Jammu and Kashmir, India.
5 Senior Resident, Department of Microbiology, Government Medical College, Srinagar, Jammu and Kashmir, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Tabindah Jahan, Reverie, Kirmani Lane No. 11, Kanitar, Naseemabad, Sadrebal, Srinagar-190006, Jammu and Kashmir, India.
E-mail: tabindah.farooq@gmail.com
Introduction
Otomycosis is a sub-acute or chronic superficial fungal infection of the external auditory canal that occurs primarily in hot dry weather of tropics and sub tropics. The disease occurs in all age groups and is generally unilateral in presentation. Different species of Aspergillus and Candida usually invade the ear canal following a primary bacterial infection although other fungal pathogens are also infrequently associated with otomycosis. Slide culture technique is often used to delineate the fungal etiology in otomycosis however the morphology is not very clear as compared to the cavity slide culture which preserves the morphology well.
Aim
To isolate various fungal agents involved in otomycosis and to compare the cavity slide culture technique with the conventional agar block slide culture for their identification.
Materials and Methods
A total of 120 cases were studied from January 2015 to June 2016. Ear discharge specimen were collected on three sterile cotton swabs. Direct examination of the specimen was carried out by Gram stain and 10% KOH mount. All specimens were inoculated on Sabouraud dextrose agar, Blood agar and Mac Conkey agar and only the ones with fungal growth were further processed. Comparison of agar block slide culture and cavity slide culture technique was done for identification of fungi. Statistical analysis was done using one-way ANOVA (analysis of variance) method and a p-value of <0.05 was taken as significant.
Results
Out of 120 fungal isolates the most common fungal isolate was Aspergillus flavus (39.2%) followed by A. niger (26.7%), A. fumigatus (15%), Penicillium (10.8%), Candida glabrata (5%) and Candida albicans (4%). Prevalence in females (65.8%) was more than males and itching (67.5%) was the most common presenting symptom. The cavity slide culture technique was found to be better in terms of proper visualisation and preservation of morphology of fungi. Growth was appreciated within 72 hours, with minimal morphological distortion of conidial attachment especially for Aspergillus and Penicillium spp. Less quantity of media was used in cavity slides which were stored for a week without the chances of contamination.
Conclusion
Cavity slide culture should be used routinely for the visualisation and identification and fungi as the morphology is better preserved and appreciated in it and the results are available in a short period of time.
Ear canal, Fungal infection, Morphological identification
Introduction
Otomycosis is a sub-acute or chronic superficial infection of the chronically inflamed external auditory canal that has been treated with antibiotic or steroid drops for a long time. The disease can extend to the middle ear cavity in case of tympanic membrane perforation [1-5]. The prevalence ranges from 9-27.2% among patients with signs and symptoms of otitis externa; while the prevalence increases to 30% among patients with discharging sinus [6]. Fungi are not the primary cause of this clinical entity rather they serve as secondary invaders superimposed on bacterial infection. Various fungi causes otomycosis, example being, hyaline saprophytic molds, dematiaceous saprophytes molds, yeasts and rarely pathogenic molds like dermatophytes. However most commonly, species of Aspergillus (A. niger, A. flavus and A. fumigatus) and Candida (C. albicans, C. parapsilosis) are recovered from such cases [7].
Factors that predispose to otomycosis include high humidity, bacterial infection, presence of cerumen, instrumentation of the ear, immunocompromised host and increased use of topical antibiotic and steroid preparations [6,8]. Otomycosis is predominantly unilateral, found in all age groups, but majority of the cases of otomycosis occur in young adults with slight preponderance in males as compared to females [9]. Cardinal features of otomycosis include pruritis, otalgia and ear discharge [4,10]. Macroscopic examination of the ear using an otoscope and microbiological examination of the ear swabs generally suffice in diagnosing this condition [10,11]. The microscopic identification of many pathogenic fungi depends on the morphological features especially the size, location and type of conidia, conidiophores and hyphae. Agar block slide culture is the mainstay for the identification of fungi however the technique is associated with problems like increased chances of contamination, long time needed for spores to develop in the agar block and distortion of morphological features. Cavity slide culture on the other hand offers less chances of contamination, relatively early visualisation of fungal structures. More importantly the morphology of the fungi remains intact which makes it easier to identify and speciate them [12]. Therefore, aim of the present study was to isolate various fungal agents in clinically suspected cases of otomycosis and to compare the conventional slide culture method with the cavity slide culture method for the identification of fungi.
Materials and Methods
This cross-sectional, prospective study was carried out in the Department of Microbiology and the Department of ENT, Vydehi Institute of Medical Sciences and Research Centre, Bengaluru, Karnataka, India, from January 2015 to June 2016 (18 months). Ethical clearance was sought from the institutes ethics committee bearing no: VIMS & RC/IEC/016/2014-15. Patients of all age groups and either sex with clinical presentation of otomycosis, who gave their consent to be a part of the study, were included and those with other ear ailments (auricular cellulitis, chronic suppurative otitis media, and ear infections due to bacteria only) were excluded from the study. In cases of mixed infection (bacterial as well as fungal), only the fungal isolate was processed further.
Sample Collection and Processing
Ear examination was done using an autoscope, the ear canal was wiped with 70% alcohol and allowed to air dry before sample collection. Patient details were recorded on pre-determined proforma. Any discharge or debris was collected on three swabs which were labeled and transported to the laboratory within 2 hours for further processing.
Swab 1 was used for Gram staining [13] and wet mount preparation using 10% potassium hydroxide (KOH) [14], swab 2 was inoculated on Blood agar and MacConkey agar and the third swab was inoculated onto two sets of Sabouraud’s Dextrose agar (SDA) which were incubated at 37°C and 25°C for 3-4 weeks. All the media was procured from Hi-Media, Mumbai, India. Any bacterial growth on Blood agar and MacConkey agar was not subjected to further processing. On SDA the following were observed: morphology of the colony, rate of growth and pigment production.
Any yeast like colony was subjected to Gram staining [13] germ tube test [13] inoculation on to Cornmeal agar (CMA), [13] and carbohydrate fermentation test [14] to identify and speciate the fungus. Lactophenol Cotton Blue (LPCB) mount [15] was made for fungal colonies on SDA resembling filamentous fungi. These were subsequently taken up for agar block slide culture and cavity side culture [12] Statistical analysis was done using one-way ANOVA (analysis of variance) method and a p-value of <0.05 was taken as significant.
Results
A total of 120 cases that fulfilled the inclusion criteria were included in the study. Higher incidence of otomycosis (61.7%) was seen in rainy season (from August to December); (n=74; 61.7%) with maximum number of patients being in the age group of 31-40 years (n=39; 32.5%). The disease was more prevalent in females (n=79; 65.8%) with a 1:3 male to female ratio. Unilateral disease was the usual presentation (n=114; 95%) with the left side being affected more (n=79; 65.8%).
Majority of the patients (n=80; 66.7%) gave a history of habitual use of unsterile wooden sticks/metal wax pickers, whereas 48.3% (n=58) of patients used oil drops. Prior antibiotic and/or steroid use was seen in 25% (n=30) of cases. Tympanic membrane perforation was seen in 15% (n=18) of the patients. The most common otoscopic finding was the presence of moist brown green mouldy material with epithelial debris (n=60; 50%). Itching was the most common symptom (n=81; 67.5%) followed by otalgia (n=79; 65.8%) and ear discharge (n=37; 30.8%). CSOM was seen in 12.5% (n=15) of the cases whereas 9.2% (n=11) patients had ASOM associated with otomycosis.
Species of Aspergillus were the most common (n=97; 80.8%) whereas the Candida species (n=10; 8.3%) were the least common fungi isolated in patients with otomycosis [Table/Fig-1]. Isolation of Aspergillus species was statistically significant (p<0.05). Comparison of agar block method and cavity slide technique for the identification of fungi and proper appreciation of morphological characteristics is shown in [Table/Fig-2]. The amount of media used as well as the processing time and risk of contamination was less for cavity slide culture method. Most importantly the morphological features of fungi were intact and better appreciated in this method than the conventional agar block technique as shown in [Table/Fig-3,4,5 and 6].
Spectrum of fungal isolates recovered from cases of otomycosis.
| Fungal species | No. of patients | Percentage | p-value |
|---|
| Aspergillus flavus | 47 | 39.2 | <0.05 |
| Aspergillus niger | 32 | 26.7 |
| Aspergillus fumigatus | 18 | 15 |
| Penicillium | 13 | 10.8 | >0.05 |
| Candida glabrata | 6 | 5 |
| Candida albicans | 4 | 3.3 |
Comparison between agar block method and cavity slide method.
| Parameter | Agar block method | Cavity slide method |
|---|
| Amount of media used | 12 mL in petri dish | 15 μL in cavity |
| Processing time | 4 days | 3 days |
| Visual clarity* | Moderate | High |
| Microscopic features** | Disturbed morphological features, not appreciated clearly | Undisturbed morphological characteristics, appreciated clearly |
| Risk of contamination*** | Yes | None |
*Visual clarity: Amount of media used in cavity slide culture is very less as such fungal elements are better appreciated
**Microscopic features: Since the amount of media used is less and the cavity slide culture entails less manipulation of the inoculated sample, microscopic features were better appreciated in it using 10x and 40x objective
***Risk of contamination: The media in cavity side culture is poured directly into cavities and sealed by the cover slip after inoculation. Hence the chances of contamination are reduced as compared to the agar block method
Note: The agar block slide culture and the cavity slide culture were seen by a trained microbiologist everyday and findings noted
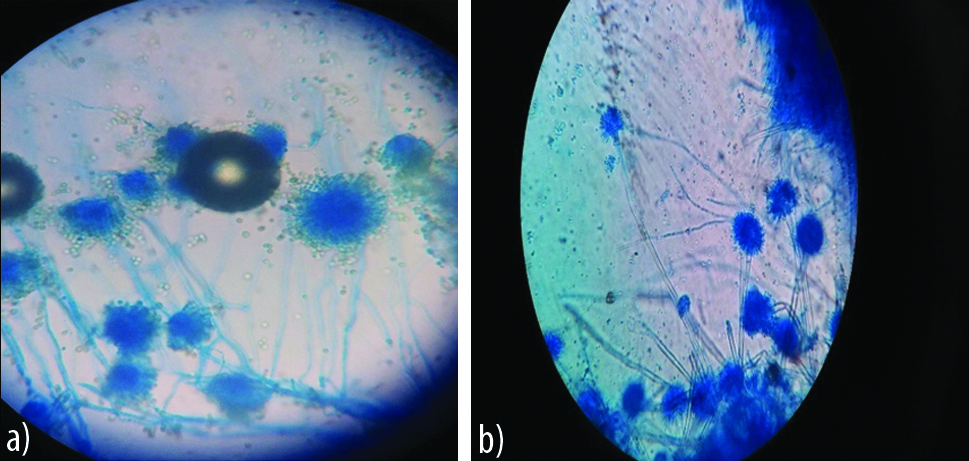
Appearance of A. flavus on agar block (left) and cavity slide culture (right).
a) Day 3: Broken conidiphore, scattered conidial heads and vesicle not visible; b) Day 2: Intact conidiophore, clear biserate philaides covering ¾ of vesicle with hyline conidia
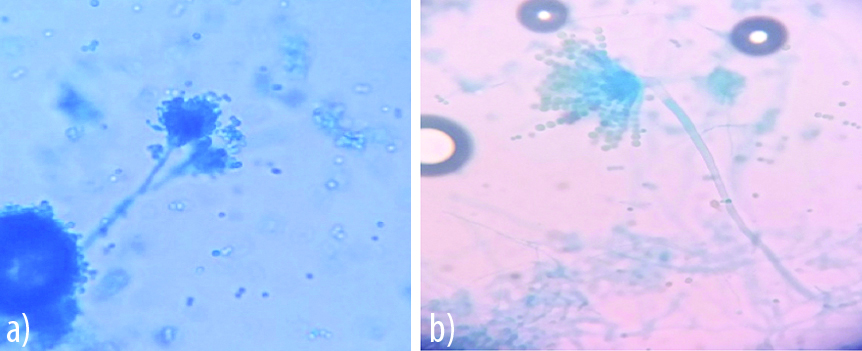
Appearance of A. fumigatus on agar block and cavity slide culture.
a) Day 3: Dbroken conidiophore, haphazard morphology.
b) Day 2: Clear vesicle, hyaline conidia covering 2/3 of vesicle, undisturbed uniseriate phialides
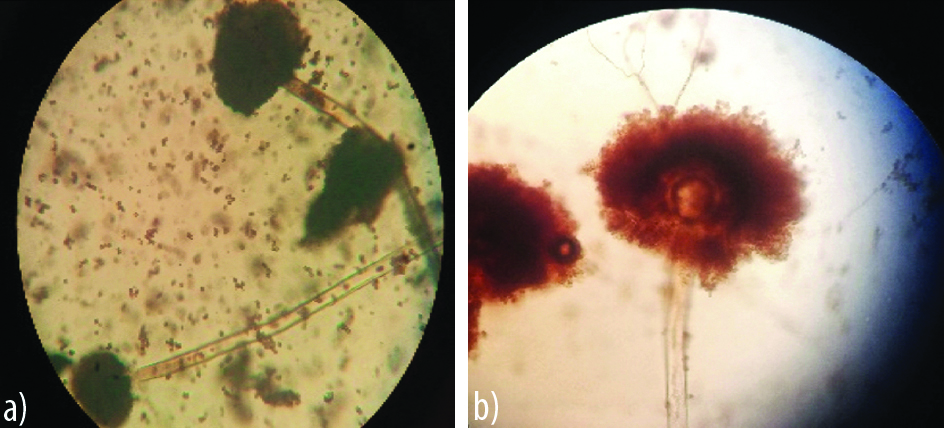
Appearance of A. niger on agar block and cavity slide culture.
a) Scattered Conidia’s disturbed conidial heads.
b) Clear large biseriate vesicle and irregular black conidia’s radiating from entire conidial head
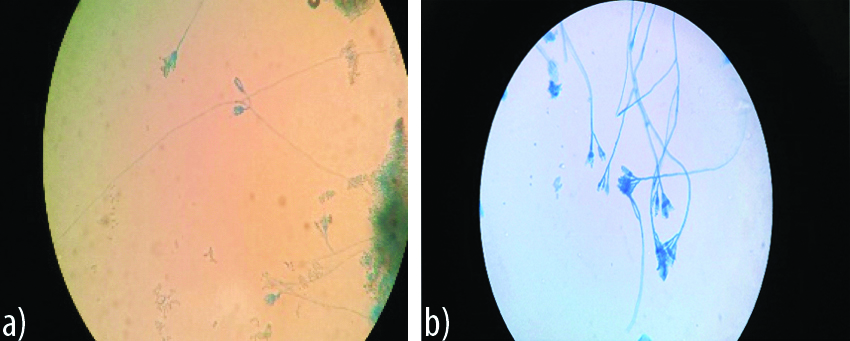
Appearance of Penicillium spp on agar block and cavity slide culture.
a) Day 4: Condiophores not well defined, few visible condial phialides not appreciated clearly
b) Day 2: Claerly defined conidiophore, smooth phialides proper conidia’s in chains (brush broder appearance)
Discussion
Otomycosis is a fairly common ear infection encountered in clinical practice. Owing to the varied microbiological flora (both bacterial and fungal) known to cause this clinical entity early diagnosis is of paramount importance to guide proper treatment. In the present study; majority of the patients were in the age group of 31 to 40 years and females were affected more than males. Similar findings have been reported by many authors [4,16,17]. Unilateral presentation and a higher incidence of otomycosis in rainy season as has been reported previously in many studies [16,18-20] was seen in our study as well. Habitual use of wooden sticks and metal wax pickers were found to be the predisposing factors causing damage to the ear canal. Likewise prior topical antibiotic use that alters defense mechanism facilitating fungal growth was also seen in many patients in our study. Findings similar to ours have been reported by other authors as well [16,18-21].
The commonest presentation of cases in this study was itching followed by otalgia and ear discharge. A study by Bhat KS et al., has reported otalgia followed by ear discharge to be the most common complaints in patients of otomycosis [22]. Agarwal P et al., in their study found that the most common presenting complaint was a blocking sensation in the affected ear followed by itching, pain, discharge and tinnitus [6]. Likewise, Gupta S et al., and Sangavi AKB et al., in their respective studies found itching to be a common presenting symptom [20,21].
Aspergillus species followed by Penicillium and Candida were isolated from all the cases of otomycosis. Among the Aspergillus species, A. flavus was the most common isolate followed by A. niger and A. fumigatus. C. glabrata was the most common species of Candida isolated from cases of otomycosis followed by C. albicans. Similar results have been reported previously by Agarwal P et al., where the authors found Aspergillus spp. (n=302; 87.3%) to be the predominant fungi isolated from the cases followed by Candida spp. (n=35; 10%) and Penicillium (n=2; 0.6%) [6]. Gupta S et al., in their study found A. niger (n=20; 51.3%), A. flavus (n=7; 17.9%), A. fumigatus and Candida spp (n=6; 15.4% respectively) to be the most common fungal isolates recovered from clinically suspect cases of otomycosis [20]. Likewise Sangavi AKB et al., have reported A. niger (n=15; 46.9%) followed by A. flavus (n=6; 18.8%) and Candida spp. (n=10; 31.3%) to be the most common fungal isolates recovered from 32 samples [21]. Penicillium species are generally considered as contaminants except Penicillium marneffei which is a pathogen in patients with AIDS or those receiving immunotherapy.
In the present study, on examination of Aspergillus and Penicillium species on the agar block culture, conidial arrangement was disturbed and very scanty whereas in comparison it was undisturbed and well preserved on the cavity slide culture. Earlier visualisation of fungal structures under both 10x and 40x objective was possible with cavity slide culture technique which was stained with LPCB using a micropipette without fully removing the coverslip. The mycelia growth occurs in a single plane along the undersurface of the cover slip in cavity slide culture thus providing clear visualisation of the fungi. With the agar block identification of fungal elements under the 40x objective was difficult due to its thickness. Also, growth and visualisation of fungi was appreciated on the third day on cavity slide culture whereas it was appreciated on the fourth day on agar block culture. Advantages of cavity slide culture include flexibility and relative ease of handling, quick turnaround time and lesser amount of media required (15 μL for cavity slide culture as compared to 12 ml for agar block culture).
Wijedsa MH et al., in their study found that using the cavity slide culture technique, earlier visualisation of structures that are required for the identification of fungi is possible [12]. In the agar block the cover slip has to be removed for staining with LPCB which invariably distorts the fungal structures and their arrangement. Also, repeated visualisation of the slide to observe different growth stages of the fungi is not possible. Furthermore, growth in the agar block takes place in various planes which can’t be better appreciated due to the thickness of the block.
Limitation
Less number of cases (n=120) was a drawback of the present study. Also, the fungal isolates recovered from the cases of otomycosis were not very diverse; hence a generalisation regarding the advantages of cavity slide culture technique over agar block slide culture cannot be made. A study that tests the cavity slide culture technique for the identification different fungal groups can make us more wiser as to the utility of this method.
Conclusion
The present study highlights the increased prevalence of otomycosis in females as compared to males with majority of the cases occurring in the rainy season. Aspergillus species was the most common fungi isolated. Also, an increased incidence of non-albicans Candida was seen. Cavity slide culture technique gave a better and early identification of the fungi as compared to agar block slide culture and should be done routinely.
*Visual clarity: Amount of media used in cavity slide culture is very less as such fungal elements are better appreciated
**Microscopic features: Since the amount of media used is less and the cavity slide culture entails less manipulation of the inoculated sample, microscopic features were better appreciated in it using 10x and 40x objective
***Risk of contamination: The media in cavity side culture is poured directly into cavities and sealed by the cover slip after inoculation. Hence the chances of contamination are reduced as compared to the agar block method
Note: The agar block slide culture and the cavity slide culture were seen by a trained microbiologist everyday and findings noted
[1]. Gokale SK, Suligavi SS, Baragundi M, Anushka D, Manjula R, Otomycosis-A clinico- mycological studyInt J Med Health Sci 2013 2:218-23. [Google Scholar]
[2]. Mugliston T, O’Donoghue G, Otomycosis-A continuing problemJ Laryngol Otol 1985 99(4):327-33.10.1017/S002221510009678X4009029 [Google Scholar] [CrossRef] [PubMed]
[3]. Fasunla J, Ibekwe T, Onakoya P, Otomycosis in western NigeriaMycoses 2008 51(1):67-70. [Google Scholar]
[4]. Pontes ZB, Silva AD, Lima E, Guerra M, Oliviera N, Carvalho M, Guerra FS, Otomycosis: A retrospective studyBraz J Otorhinol 2009 75(3):367-70.10.1590/S1808-86942009000300010 [Google Scholar] [CrossRef]
[5]. Pradhan B, Tuladhar NR, Amatya RM, Prevalence of otomycosis in outpatient department of otolaryngology in Tribhuvan University Teaching Hospital, Kathmandu, NepalAnn Otol Rhinol Laryngol 2003 112(4):384-87.10.1177/00034894031120041612731637 [Google Scholar] [CrossRef] [PubMed]
[6]. Agarwal P, Sumitra Devi LS, Otomycosis in a rural community attending a tertiary care hospital: Assessment of risk factors and identification of fungal and bacterial agentsJournal of Clinical and Diagnostic Research 2017 11(6):DC14-DC18.10.7860/JCDR/2017/25865.1006828764159 [Google Scholar] [CrossRef] [PubMed]
[7]. Gharaghani M, Seifi Z, Mahmoudabadi Z A, Otomycosis in Iran: A reviewMycopathologia 2015 179(6):415-24.10.1007/s11046-015-9864-725633436 [Google Scholar] [CrossRef] [PubMed]
[8]. Ho T, Vrabec JT, Yoo D, Otomycosis: Clinical features and treatment implicationsOtolaryngol Head Neck Surg 2006 135:787-91.10.1016/j.otohns.2006.07.00817071313 [Google Scholar] [CrossRef] [PubMed]
[9]. Moharram AM, Ahmed HE, Nasir SAM, Otomycosis in Assuit EgyptJ Bas App Mycol 2013 4:01-11. [Google Scholar]
[10]. Dhingra PL, Dhingra S, Diseases of the ear nose and throat 2010 5th editionNew DelhiElsevier [Google Scholar]
[11]. Chandra J, Medical Mycology 2009 3rd editionMehta Publications [Google Scholar]
[12]. Wijedasa MH, Liyanapathirana LVC, Evaluation of an alternative slide culture technique for he morphological identification of fungal speciesSri Lanka J Infect Dis 2012 2(2):47-52.10.4038/sljid.v2i2.4070 [Google Scholar] [CrossRef]
[13]. Metwally A, Ahmad G, Hanan MN, Epidemiology causative agents and risk factors affecting human otomycosis infectionsTurk J Med Sci 2015 45:820-26.10.3906/sag-1407-17 [Google Scholar] [CrossRef]
[14]. Joy MJ, Agarwal MK, Samant HC, Gupta OP, Sharma BM, Mycological and bacteriological studies in otomycosisIndian J of Otolyrangol 1980 32:71-75. [Google Scholar]
[15]. Rowlands S, Devalia H, Smith C, Hubbard R, Dean A, Otitis externa in UK general practice: A survey using the UK general practice research databaseBr J Gen Pract 2000 51(468):533-38. [Google Scholar]
[16]. Prasad SC, Kotigadde S, Shekhar M, Thada ND, Prabhu P, Souza TD, Primary otomycosis in Indian subcontinent, predisposing factors, microbiology and classificationInt J Microbiol 2014 :01-09.10.1155/2014/63649324949016 [Google Scholar] [CrossRef] [PubMed]
[17]. Adoga AS, Iduh AA, Otomycosis in JOS: Predisposing factors and managementAfr J Med Sci 2014 14(1):209-13. [Google Scholar]
[18]. Mohammad S, Ujjan ID, Otomycosis: A clinicopathological studyJournal of Surgery Pakistan 2016 21(4):145-48.10.21699/jsp.21.4.8 [Google Scholar] [CrossRef]
[19]. Vaidyanath V, Sreenath K, Chandra SK, Ratan S, Predisposing factors and clinical study of otomycosis in the southern sub-tropical regions of IndiaRGUHS J Med Sciences 2012 2(4):212-16. [Google Scholar]
[20]. Gupta S, Mahajan B, Prevalence and demographic profile of patients presenting with otomycosisJK Science 2015 17(3):138-42. [Google Scholar]
[21]. Sangavi AKB, Peerapur B, Gummadi N, Clinico-mycological study of otomycosis in Raichur, Karnataka: A hospital based studyInt J Otorhinolaryngol Head Neck Surg 2018 4(1):133-36.10.18203/issn.2454-5929.ijohns20175624 [Google Scholar] [CrossRef]
[22]. Bhat KS, Kulal B, Meundi M, Kotigadde S, A microbiological study of otomycosisIndian J Microbial Research 2017 4(1):118-25. [Google Scholar]