The formation of incipient carious lesion arises from a process of demineralization and remineralization that occurs in the dental enamel as a consequence of the action of microorganisms in the biofilm generated by carbohydrate metabolism. Furthermore, the presence of fluoride in saliva and other remineralizing agents enables the remineralization of previously demineralized enamel. In an in vitro model of pH cycling, ten Cate JM et al., demonstrated the ability of fluoride to enable the remineralization of enamel [1]. By means of this process, fluorine is used in dentistry to revert dental demineralization by increasing the gradient of fluorine, within the oral environment, from both intrinsic and extrinsic sources. The active and passive mechanisms of mineral transportation are first stimulated in the environment and then take effect in the tooth. When fluorine comes into contact with the dental structure, it interacts with the OH- groups of hydroxyapatite (HA), transforming HA into Fluoro-hydroxyapatite (FHA) [2].
Acidulated phosphate fluoride (APF) is widely used in preventive dentistry because its mechanism of action involves the demineralization of the enamel surface and the increased availability of calcium ions [12,13], which interact with fluoride to produce a precipitate of calcium fluoride. This fluoride gel (1.23%) is immediately placed on the dental surface, producing a recrystallization phenomenon in the dental enamel via the incorporation of fluoride within the crystal lattice of HA, converting it into FHA and thus making dental enamel highly resistant to caries [14].
In preventive dentistry, fluoride is applied to the outer surface of the dental enamel to prevent the development of carious lesions. The benefits of its use has been overstated without discussing the differences among concentrations and type of vehicle (gel, varnish, and solution), because they are the most commonly used in paediatric dental practice.
The term microhardness refers to the resistance of a material to penetration by an indenter applied withloads below 1 kg of force (kg/f) [15]. The Vickers method for testing hardness is widely used in dentistry because it can evaluate the level of mineralization in a dental substrate [14]. pH cycling involves alternating the application of solutions involved in demineralization and remineralization at constant time intervals in order to simulate pH cycles in the oral environment, providing in vitro models that reproduce the caries process for research purposes. Demineralization and remineralization studies have increased the knowledge about the complex mechanisms that cause a loss or uptake of mineral ions. Carefully designed, such tests can provide insight into the rate-limiting steps of ion diffusion, crystal growth, or crystal formation, with the ultimate goal of formulating effective methods for interfering with the caries process. Cariogenic challenge and possible repair are usually of limited duration in the mouth, in contrast to most in-vitro models. Following the consumption of fermentable carbohydrates, the pH level in the plaque shows a sharp decline, followed by a slow and gradual return to physiological pH level. Throughout the day, this results in a series of 30-minute pH depressions, interspersed with ‘resting’ periods. As the solubility of all calcium phosphate minerals is pH-dependent, this pH cycling leads to a dissolution and reprecipitation of tooth mineral [16-18]. This study aimed to evaluate the remineralization of dental enamel by measuring the effect of the application of the three most commonly used fluorinated compounds in paediatric dentistry- sodium fluoride, acidulated phosphate fluoride and silver diamine fluoride- on the microhardness of enamel under pH cycling conditions. The null hypothesis of the study was that no remineralizing effect on dental enamel would be found after applying the different fluorinated compounds under cyclic pH conditions.
Materials and Methods
Specimens and Preparation of Sample Group
This in vitro study was done on 3rd molars extracted from patients attending, the Maxillofacial Surgery Clinic of the Division of Postgraduate Studies and Research of the Faculty of Dentistry, at the National Autonomous University of Mexico. The present study was approved by the Research and Ethics Committee at the School of Dentistry and was in accordance with the World Medical Association Declaration of Helsinki. All the third molars were obtained over a period of three months. Informed consent was obtained from patients undergoing dental extractions procedures, all of whom were between 17 and 22 years old.
The study was performed on 60 third molars with intact anatomical crowns and without recently treated structural defects (the molars that presented healthy tooth enamel). All third molars were sectioned longitudinally in a mesiodistal direction with a diamond disc (Brasseler™ diamond, California, US) under constant irrigation [Table/Fig-1], after which, the 120 work surfaces obtained were filled with pink wax in the internal area of the tooth (the pulp chamber and the root canals) in order to obtain a smooth surface [Table/Fig-2].
Third molar after mesiodistal sectioning.
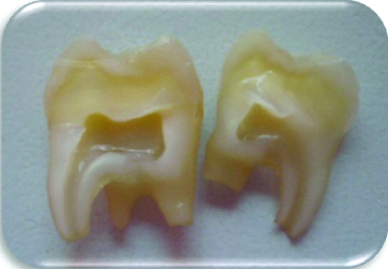
120 work surfaces that were filled with pink wax in the internal area of the tooth to obtain a smooth surface.

Periodontal debris was removed prior to the tooth being sectioned, while the molars were stored in deionized water until the experimental tests were performed. In accordance with ten Cate JM et al., [19], prophylaxis was undertaken with non-fluoridated prophylactic paste (QOM™). A working area was created by covering the root and crown with acid-resistant varnish (Revlon™ different colors, New York, US), leaving a 3×6 mm area in each sample at the center of the lingual or buccal surface of the crown. The study comprised four groups, with 30 samples included in each group [Table/Fig-3].
Conditioning of the working surface (buccal and lingual surface) with prophylactic paste without fluoride and work surfaces (buccal and lingual) with the window for the application of the corresponding fluorinated compound.
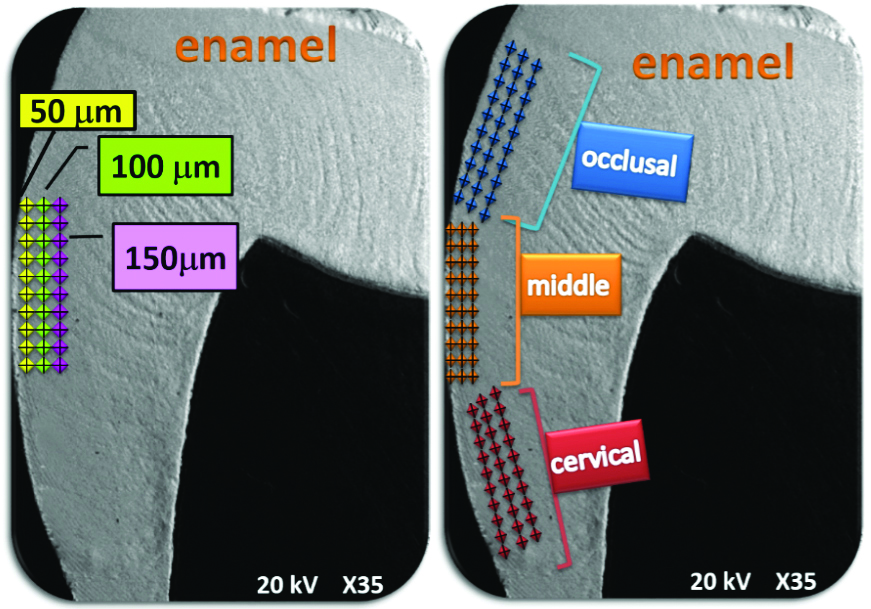
Microhardness testing was carried out with the Vickers microhardness tester using a standard load of 25 g and a dwell time of 20s, with 90 notches applied per sample [20]. The notches were applied perpendicular to the most external enamel surface near the enamelo-dentinal junction in an occlusal, middle and cervical direction at depths of 50, 100 and 150 microns [Table/Fig-4].
Schematic representation of the place of the indentations.
Demineralized Lesion (initial lesion)
ten Cate JM et al., designed a pH cycling model to study mineral loss and gain in artificial enamel lesions, generating a model which is the best reproduction of in-vivo conditions. As the work surfaces were healthy, initial lesions were made with demineralizing solution, after which 120 work surfaces (buccal and lingual faces) were immersed in a demineralizing solution (2.2 mM CaCl2, 2.2 mM NaH2PO4, and 0.05 M acetic acid) for 96 hours at 37°C [19], while the pH was adjusted, using a solution buffer, to 4.4 with 1M KOH [21]. Once the 96 hours had elapsed, all demineralized surfaces were randomised and experimental groups were formed to receive the indicated treatment: Group I- SDF (n=30) (SDF-Saforide™); Group II- DSF (n=30) (DSF-Fluor Protector™); Group III- APF (n=30) (APF-Sultan-Health™); and, Group IV- Control Group (CG) (n=30), which received no treatment.
The following treatment process was applied:
The surfaces of the tooth were removed from demineralizing solution
All work surfaces were rinsed with deionized water
The fluoride compounds were placed on each surface using their respective brush
The fluorinated compound was left on the work surfaces for 60 seconds, with the time taken on a stop watch, in accordance with the manufacturer’s instructions.
Once the time had elapsed, the sample was placed in there mineralizing solution in order to continue the pH cycling.
The pH cycling sequence is given as follows:
a) 21 hours in remineralizing solution (1.5 mM CaCL2, 0.9 mM NaH2PO4 and 0.15 mM KCL) with the pH adjusted, with a solution buffer, to 7.0 with 1M KOH.
b) 3 hours in demineralizing solution (2.2 mM Ca, 2.2 mM P, and 5.0 mM CH3COOH) with a pH of 4.4.
c) 60-second application of the fluorinated compound (SDF, DSF or APF). Between the application of the demineralizing and remineralizing solutions, the working surface was rinsed with deionized water. The composition of the demineralizing solution was 2.2 mM Ca, 2.2mM P, and 5.0 mM CH3COOH, with a pH of 4.4, while the composition of the remineralizing solution was 1.5 mM CaCL2, 0.9 mM NaH2PO4 and 0.15 mM KCL, with the pH adjusted with a solution buffer to 7.0 with 1M KOH. The solutions were prepared every third day. After 5, 10 and 15 days, the pH cycling solutions (those used for both remineralization and demineralization) were removed from the working surfaces to enable the Vickers microhardness tests. Before the microhardness test was performed, the surfaces were immersed in deionized water at 37°C.
Vickers Microhardness
The remineralizing and demineralizing solutions were prepared daily. After 5, 10 and 15 days, 10 working surfaces from each group were removed at random in order to determine their microhardness. The working surfaces were placed on a ruler in order to make the longitudinal cuts required to obtain the dental sub-surface [Table/Fig-5], after which, the working surfaces were placed in acrylic moulds to enable transfer to the microdurometer [Table/Fig-6].
Work surfaces placed on a ruler to be cut longitudinally and get the subsurface dental.
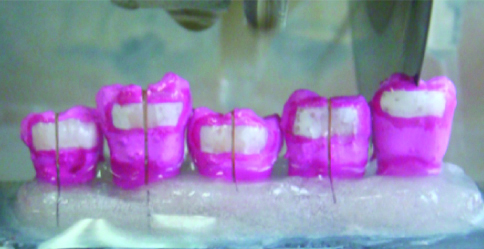
Work surface already cut longitudinally and placed in acrylic moulds. Assembly of the longitudinally cut work surfaces and mounted in acrylic moulds to determine the microhardness of the subsurface dental enamel.
The exposed section of the working surface of each sample was found using a 400X objective lens in the microdurometer, with the penetrating Vickers indentor applied perpendicularly to the tooth surface with a constant load of 25 gf. The Vickers microhardness was calculated using the formula, Vertical Diagonal (VD) + Horizontal Diagonal (HD) = Total Diagonal (TD), where VD is the measure of the vertical diagonal and HD is the horizontal diagonal measurement. The VHN was determined by locating TD in the Vickers microhardness tables (25 gf) [Table/Fig-5].
Statistical Analysis
The microhardness data was reported as the mean for each experimental group and statistically analysed via a one-way analysis of variance (ANOVA) in which the mean microhardness of the tooth enamel surface was compared for the three treatment groups at depths of 50, 100 y 150 μm. Post-hoc Tukey’s tests were then used to observe the differences between the three treatment groups, obtaining a significance level of p<0.05. The analysis was performed using Stata V.14 software.
Results
[Table/Fig-7] present the VHN means after 5, 10 and 15 days. After five days of treatment, the three experimental groups (SDF, DSF and APF) were compared at 50, 100 and 150um and in terms of occlusal, middle and cervical notch positions. Moreover, after 10 days of treatment, high VHN values (119, 99.5, and 140) were found at an occlusal notch position at depths of 150μm for the SDF, DSF and APF groups, respectively, while statistically significant differences were found for all three experimental groups (SDF p=0.016, DSF, p=0.043 and APF p=0.035). After 15 days of treatment, the DSF group recorded high VHN values at depths of 50, 100 and 150 μm, with statistically significant differences. The SDF and DSF groups also showed statistically significant differences in VHN values at depths of 50, 100 and 150 μm (SDF p=0.023 and DSF p=0.031).
Microhardness (VHN) in the three groups of treatment with different remineralization time periods and depths 50, 100 y 150 μm with respect to the surface of the tooth enamel.
| | 5 days | 10 days | 15 days |
|---|
| Group | | 50 μm | 100 μm | 150 μm‡ | 50 μm | 100 μm | 150 μm‡ | 50 μm | 100 μm | 150 μm‡ |
|---|
| | Mean | Mean | Mean |
|---|
| CG | Occlusal | 143.0 | 137.0 | 148.0* | 109.0 | 110.0 | 103.0* | 76.0 | 89.9 | 81.8* |
| Medio | 140.0 | 145.0 | 162.0* | 110.0 | 119.0 | 117.0* | 70.7 | 85.4 | 79.1* |
| Cervical | 126.0 | 179.0 | 150.0* | 111.0 | 121.0 | 118.0* | 86.1 | 84.6 | 84.6* |
| SDF | Occlusal | 98.4 | 98.0 | 98.0* | 83.2 | 108.0 | 119.0** | 101.0 | 101.9 | 108.0** |
| Medio | 86.0 | 86.1 | 104.0* | 116.1 | 117.1 | 121.0** | 114.1 | 118.1 | 122.0** |
| Cervical | 40.3 | 92.4 | 96.6* | 114.0 | 122.0 | 127.0** | 105.0 | 107.0 | 117.0** |
| DSF | Occlusal | 84.6 | 74.8 | 83.9* | 88.4 | 97.2 | 99.5** | 124.0 | 130.0 | 140.0** |
| Medio | 87.6 | 76.0 | 77.9* | 57.9 | 61.7 | 71.2** | 114.0 | 121.0 | 127.0** |
| Cervical | 117.0 | 118.0 | 94.0* | 57.5 | 59.5 | 67.1** | 104.0 | 123.0 | 126.0** |
| APF | Occlusal | 105.0 | 131.0 | 116.0* | 124.0 | 130.0 | 140.0** | 104.0 | 106.0 | 108.0** |
| Medio | 133.0 | 114.0 | 119.0* | 114.0 | 121.0 | 129.0** | 99.3 | 111.0 | 116.0** |
| Cervical | 131.0 | 209.0 | 119.0* | 135.0 | 124.0 | 128.0** | 100.0 | 110.0 | 115.0** |
VHN: Vickers hardness number; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride; One-way analysis of variance (ANOVA) The comparison was made between 50, 100 and 150 μm and also among the between occlusal, medio and cervical group; *p>0.05, **p<0.05 Statistically significant; ‡ Depth with respect to the surface of the tooth enamel (50 μm, 100 μm, 150 μm)
[Table/Fig-8] present the results of the post-hoc Tukey’s Honest Significant Difference (HSD) test, which revealed statistical differences among the groups (p≤0.05). Although significant differences were determined after five days of treatment, no significant difference was observed between the CG and APF groups. When this comparison was undertaken after 10 days of treatment, no significant differences were found between either the CG and APF groups or the SDF and DSF groups. After 15 days of treatment, statistical differences were determined among the groups, but these were not significant for the DSF and SDF groups.
Comparison of microhardness per day and treatment group, results of Tukey’s HSD post-hoc test.
| Day | Group (I) | Group (J) | Mean Difference (I-J) |
|---|
| 5 | SDF | DSF | -27.46* |
| APF | 72.57* |
| CG | -65.07* |
| DSF | SDF | 27.46* |
| APF | -45.11* |
| CG | -37.61* |
| APF | SDF | 72.57* |
| DSF | 45.11* |
| CG | 7.50 |
| CG | SDF | 65.07* |
| DSF | 37.61* |
| APF | -7.50 |
| 10 | SDF | DSF | -7.52 |
| APF | -52.39* |
| CG | -55.52* |
| DSF | SDF | 7.52 |
| APF | -44.87* |
| CG | -48.00* |
| APF | SDF | 52.39* |
| DSF | 44.87* |
| CG | -3.13 |
| CG | SDF | 55.52* |
| DSF | 48.00* |
| APF | 3.13 |
| 15 | SDF | DSF | 5.58 |
| APF | -52.33* |
| CG | 40.61* |
| DSF | SDF | -5.58 |
| APF | -57.91* |
| CG | -46.19* |
| APF | SDF | 52.33* |
| DSF | 57.91* |
| CG | 11.72* |
| CG | SDF | 40.61* |
| DSF | 46.19* |
| APF | -11.72* |
*Statistically significant p<0.05; CG: Control group; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride
[Table/Fig-9] shows the results of the statistical analysis applied, which considered each treatment as a comparative variable (Tukey’s HSD test), with the results enabling the identification of both statistical differences within each group and differences among the different number of days of treatment (5, 10 or 15). The DSF group was unique in its results, with significant statistical differences found for all observations undertaken, while the SDF group was statistically different only when Day 15 was compared with the Day 5 results for other groups. The APF group was revealed to be different in each experimental comparison except day 10 and 15, while statistically significant differences were found for the CG group at all times.
Comparison of microhardness between different treatment groups, results of Tukey’s HSD post-hoc test.
| Group | Day (I) | Day (J) | Mean Difference (I-J) |
|---|
| CG | 5 | 10 | 13.27 * |
| 15 | 29.51 * |
| 10 | 5 | -13.27 * |
| 15 | 16.24 * |
| 15 | 5 | -29.51 * |
| 10 | -16.24 * |
| APF | 5 | 10 | 23.90 * |
| 15 | 25.29 * |
| 10 | 5 | -23.9 * |
| 15 | 1.39 |
| 15 | 5 | -25.29 * |
| 10 | -1.39 |
| DSF | 5 | 10 | 23.66* |
| 15 | 38.09* |
| 10 | 5 | -23.66* |
| 15 | 14.43* |
| 15 | 5 | -38.09* |
| 10 | -14.43* |
| SDF | 5 | 10 | 3.72 |
| 15 | 5.05* |
| 10 | 5 | -3.72 |
| 15 | 1.33 |
| 15 | 5 | -5.05* |
| 10 | -1.33 |
*Statistically significant p<0.05; CG: Control group; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride
Discussion
At present, in-vitro study evaluated tooth demineralization and remineralization at three different locations in the enamel (occlusal, middle and apical enamel) by means of Vickers microhardness measurements. This research used three different fluoride compounds, SDF, DSF and APF, to perform the remineralization. The APF is effective since from day 5 it increases its VHN values to more than 100 and keeps them constant at 10 and 15 days, although at day 15 the value of VHN decreases slightly. On the other hand, the DSF increased its VHN values until day 15, reaching VHN values above 100. Therefore, we can say that the APF produces a greater remineralization in a shorter time in comparison with the DSF. Mei ML et al., showed that SDF reacts with calcium and phosphate ions, producing the precipitation of fluoro-hydroxyapatite with reduced solubility, which could be one of the main factors behind the arrest of carious lesions treated with SDF [22]. Several studies have also reported that DSF and APF have a preventive effect on the development of caries [23,24].
The physical property of materials, hardness, can be studied with a reproducible methodology, with its values depending on the notch (or indentation) of hardness test used. The micro-indentations measured in the present study were produced using a Vickers indentator. The VHN test is a simple, fast and non-destructive method and is, according to the literature [15], the most commonly used method to measure the microhardness of different materials. Strassler HE et al., performed a study to determine the microhardness of human and bovine teeth using the Knoop method and failed to find a significant statistical association (p<0.001) between species. The method they used was found to be indeterminate because the values were equivalent to those obtained with the Vickers method [25].
The present research revealed an increase in microhardness, after 10 and 15 days of treatment, in the three experimental groups, finding significant differences mainly in the occlusal and apical areas. Sivapriya E et al., reported an increase in microhardness after seven, 14 and 28 days post-remineralization [26], while Molaasadolah F et al., reported an increase in microhardness both before and after the application of fluoride varnish (from both the Duraflor and Ariadentbrands) [27]. Thus, the application of fluoride over different periods of time could increase the microhardness of enamel in the long term.
Another study, however, evaluated the in-vivo resistance of dental enamel exposed to acid attack both before and after the application of different fluoride agents (APF, NaF, and SDF), concluding that the treatments produced an increased resistance to acid attack. At the same time, the results also indicated that the group treated with SDF exhibited a greater effect on the dental enamel [8]. The resistance of the enamel was determined by direct observation rather than objective means, the same manner in which we observed the effects of fluorinated compounds on the dental surface.
The literature on this area presents many in-vitro studies that have examined how the demineralization process affects dental enamel [28,29]; however, as researchers have used a variety of methods to expose the dental surface to different acid solutions, it is difficult to compare their results. In order to understand more accurately the physiological conditions simulated, researchers have developed pH cycling systems [19], in which demineralization episodes are alternated with periods of remineralization. The present research was performed with the pH cycling model proposed by ten Cate, because it resembles in-vivo conditions [1].
Seppä L et al., used polarised light microscopy to assess the influence of vehicles on fluoride compound, APF gel, and NaF varnish, concluding that fluoride compound used in varnish form is as effective as fluoride used in gel [30]. We found a statistical difference related to the vehicle of fluoride administration because the APF (gel) achieved better VHN values than either the DSF solution or the DSF varnish, a result that we consider was due to the different instruments used to determine the same phenomena.
Chu and Lo evaluated the efficiency of DSF and NaF varnish for the treatment of caries [4], concluding that a single annual application of DSF was more effective for treating caries than NaF varnish [4], a result explained by the precipitation of silver phosphates on dental surfaces when DSF was applied [8]. In addition, in case this mechanism was arresting the development of caries, the Knoop hardness test was used to determine the effect on hard surfaces, in this instance, the dentine. Dental enamel is affected by changes in the oral environment and reduced pH near the enamel surface generated by the bacterial metabolism. This leads to demineralization as a result of the alteration of the inorganic component (Ca5(PO4)3OH), thereby modifying physical properties such as hardness [31]. A homeostatic mechanism occurs when available Ca2+ and (PO4)3- ions interact with the dental structure, improving the physical properties of demineralized tissues [17]. The present study determined microhardness after the exposure of enamel surfaces to alternated pH cycling conditions and daily treatment with different fluoride compounds. Our results indicate that the group treated with APF presented higher VHN values in shorter time than the other groups, which could be associated with the compound vehicle because daily exposure to APF gel may generate more available Ca2+ and (PO4)3- ions than either the varnish or solution. In contrast, the DSF and SDF microhardness values recorded at the end of the experiment were different to those reported in the literature. Several reports have mentioned higher VHN values compared with DSF; however, the polymer layer that formed on the enamel surface was not considered, even if DSF was applied on a daily basis, because the thickness of the layer increased over time. This phenomenon explains the lower values observed in the group treated with DSF.
Limitation
One of the limitations of the present study was that remineralization was physically characterised solely by means of the microhardness test; however the effect of said remineralization should be determined by chemical characterization using Raman spectroscopy.
Conclusion
The present study found that the use of remineralizing agents such as SDF, DSF and APF increases the microhardness of the enamel surface. Therefore, these different fluorinated compounds are useful in effectively reducing white stains on the enamel and remineralizing the incipient carious lesions. The different fluorinated compounds (vehicles) should continue to be applied in the general population, because the failure to do so may result in demineralization and the loss of dental enamel continuity. In this sense, the best fluorinated compounds found in the present investigation was APF, since it presents a faster remineralization in less time (from day 5), followed by the DSF, since both fulfill the objective of remineralizing the tooth enamel. If the APF is applied early in carious lesions in children, it stops the demineralization of the enamel in less time.
VHN: Vickers hardness number; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride; One-way analysis of variance (ANOVA) The comparison was made between 50, 100 and 150 μm and also among the between occlusal, medio and cervical group; *p>0.05, **p<0.05 Statistically significant; ‡ Depth with respect to the surface of the tooth enamel (50 μm, 100 μm, 150 μm)
*Statistically significant p<0.05; CG: Control group; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride
*Statistically significant p<0.05; CG: Control group; SDF: Silver diamine fluoride; DSF: Difluoride silane; APF: Acidulated phosphate fluoride