Dental impressions contribute to an important step to get a perfect cast, as the aim of an impression is to produce a dimensionally stable “negative” to serve as the cast mold. The impression materials should reproduce the static and oral structures accurately [1]. Among the options of these materials, dentists have tended to use vinyl polysiloxane because of their improved physical and mechanical properties and good patient acceptance.
To prevent cross-contamination, impressions should be properly disinfected after removing from the mouth, since they are always contaminated with saliva, frequently with blood and bacterial plaque [2]. Manipulation of these contaminated impressions may contribute to the dissemination of causative microorganisms of infectious and contagious diseases [3,4]. At present, there is a variety of chemical products sold as agents suitable for disinfecting dental impressions. To be efficacious, a successful disinfection must maintain the physico-chemical properties of the impression materials and also not interfere negatively in the obtainment of stone casts [5].
Among the chemical disinfectants available, glutaraldehyde has been widely used because it presents compatibility with many impression materials and has also demonstrated a bactericidal and fungicidal effect and virucidal action [6,7]. Nevertheless, glutaraldehyde releases toxic vapors, irritants and allergens that cause irritation to the eyes, nose and throat, allergy, contact dermatitis, asthma and rhinitis [8].
A safe alternative to glutaraldehyde, as an effective disinfectant, is the peracetic acid [9], which is a powerful microbicidal agent used for high-level disinfection in hospitals. According to the FDA’s Classification of Sterilising and Disinfecting Chemical Liquids published in the Federal Register, the peracetic acid is a mild, non-toxic and non-allergenic irritant, being recommended for high-level disinfection processes [10]. However, its use to disinfect dental impressions is still scarcely mentioned in the literature.
In addition to the impression materials and the chemical agents used during the disinfection process, professionals should consider the technique of choice as well. Therefore, in order to obtain decontaminated stone casts, these three factors should be interdependent factors. As already mentioned, the immersion impression technique has been the widely applied; however, studies have described a new method of disinfection by ultrasonic nebulization for dental impressions [11-14].
The nebulization process has been used for medical treatment purposes. Nebulization is characterised by dispersion of liquid into the air [12]. Ultrasonic nebulizers use a piezoelectric crystal that emits ultrasonic waves to produce aerosol. This method requires only around 10 mL of the disinfection solution for each cycle [12]. When used for disinfection, the interaction of the effect of ultrasonic nebulization and the chemical agent may potentiate its action; ultrasonic nebulization with 2% glutaraldehyde has shown higher microbicidal activity when compared to the immersion method using the same chemical agent [13].
Many studies have evaluated comparatively different mold disinfection techniques [15-18]. However, despite microbiological evaluation of the ultrasonic nebulization effect with peracetic acid and glutaraldehyde on disinfection methods of dental impressions, literature information about the effect of the nebulization method on the dimensional precision of dental impressions is scarce [13]. In addition, there were no studies found evaluating the accuracy of VPS immersed in peracetic acid solution.
Materials and Methods
The study was conducted between January and June 2010 in Cascavel Campus of State University of Western Paraná (UNIOESTE), Paraná, Brazil, The sample was calculated using a family F probability, with a repeated families design, with interaction within and among the factors. The effect size of 0.18, type 1(α) error of 0.05, and analysis power of 0.90 chosen resulted in 40 sample units, with 8 samples per experimental group (n=8). G Power software (version 3.1.9.2, University of Düsseldorf, Germany) was used for sample calculation. Since this was an in-vitro study, ethical clearance was not found necessary to be obtained. Thus ethical clearance was not obtained. The materials used in this study are shown in [Table/Fig-1].
Materials used and manufacturers.
| Material | Commercial brand-manufacturer |
|---|
| Vinyl polysiloxane | Aquasil Easy Mix Putty, Dentsply Caulk, Milford, USA. |
| Aquasil Ultra LV, Dentsply Caulk, Milford, USA. |
| 0.2% peracetic acid | Sterilife, Lifemed Ind. Equip. Art. Med. Hosp. S.A., Pelotas, RS, Brazil. |
| 2% glutaraldehyde | Glutaron II, Ind. Farm. Rioquimica Ltda., Sao Jose do Rio Preto, SP, Brazil. |
| Type IV gypsum | Durone IV, Dentsply Ind. e Com. Ltda, Petropolis, RJ, Brazil. |
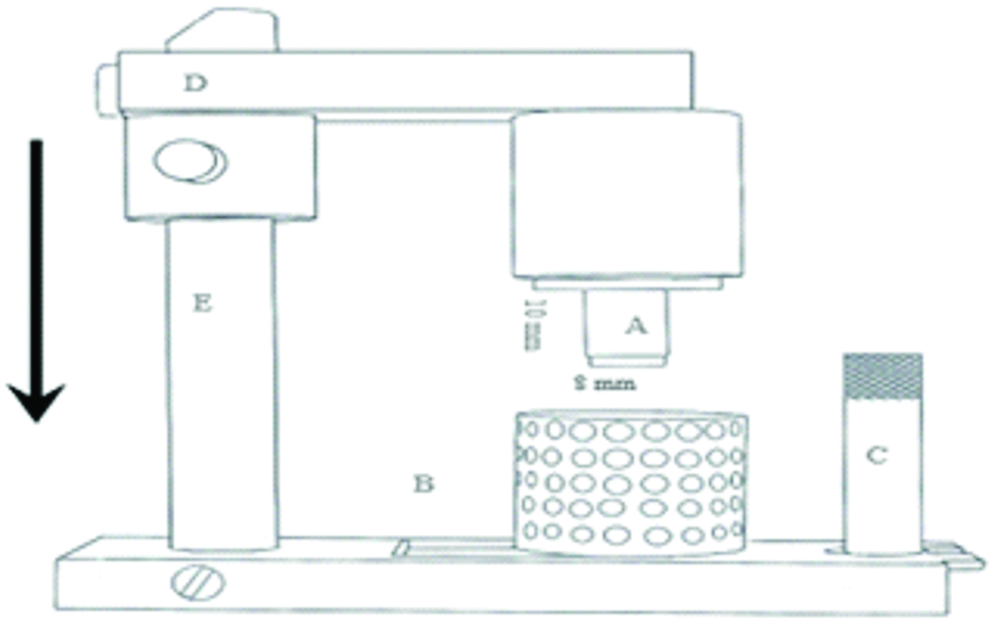
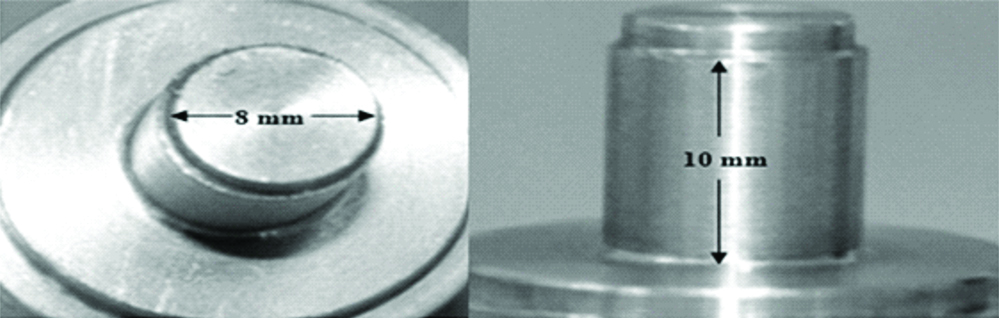
The accuracy of impressions submitted to different disinfection techniques was evaluated by means of stone casts dimensions obtained from a stainless steel pattern model [14-18]. This pattern model was used to obtaining cylindrical samples, with 8 mm in diameter and 10 mm in height [Table/Fig-2]. Joined to it, there was a mobile device that ran along a vertical bar, allowing the cylinder to meet with a tray containing the impression material in a standardised thickness. The trays were prepared with 20-mm-height and 20-mm-internal diameter. This mobile device allowed standardisation of pressure used during the act of taking the impression [Table/Fig-2].
Diagram illustrating the stainless steel pattern apparatus used for the impression. A- pattern model, B- impression tray, C- pin for fixing tray, D- mobile device, E- fixed vertical bar.
In addition to the technique for disinfection by immersion for 10 minutes, the technique for disinfection by ultrasonic nebulization was also evaluated. This technique was performed using an ultrasonic nebulizer (Pulmonosic Star II, Soniclear, São Paulo, São Paulo, Brazil), set at an ultrasonic frequency of 2.4 MHz, nebulization rate of 1.25 cc/min, and the disinfectant solution mist was guided into a transparent plastic box (20×20×25 cm). The samples in the box were disinfected by means of ultrasonic nebulization and kept for 10 minutes until the fog in the box reached saturation [10]. The solutions used were 0.2% peracetic acid (Sterilife, Lifemed Ind. Equip. Art. Med. Hosp. S.A.) and 2% glutaraldehyde (Glutaron II, Ind. Farm. Rioquimica Ltda).
The impressions were made with vinyl polysiloxane (Aquasil, Dentsply Caulk, Milford, USA.) by means of the one-step impression technique, made with the putty and light-body materials simultaneously, because this technique is one of the most used in clinical dentistry. The impressions were allowed to polymerize on the stainless steel pattern model for 12 minutes [19] and remained in a controlled environmental condition at 23±1°C. The putty consistency VPS material was proportioned and manipulated based on the manufacturer’s instructions. The light-body material was used with an automatic mixing syringe. The 40 impressions obtained were washed under running water for 10 seconds and then randomly submitted to one of the 5 experimental conditions (n=8), according to [Table/Fig-3].
| Groups | Disinfection technique |
|---|
| I | Control-impressions without any disinfection. |
| II | Immersion in 2% glutaraldehyde for 10 minutes. |
| III | Immersion in 0.2% peracetic acid for 10 minutes. |
| IV | Nebulization with 2% glutaraldehyde for 10 minutes. |
| V | Nebulization with 0.2% peracetic acid for 10 minutes. |
After disinfection period, the impressions were again washed under filtered running water for 10 seconds and dried with air jets. After the period of 1 hour, in order to favor the release of hydrogen, the impressions were filled with type IV gypsum (Durone IV, Dentsply Ind. e Com. Ltda, Petropolis, RJ, Brazil.) to obtain the stone casts. The gypsum was manipulated in accordance with the manufacturer’s instructions. To mix the gypsum, a mechanical vacuum mixer (A 300, Polidental, Cotia, São Paulo, Brazil) was used for 60 seconds. The gypsum was poured into the impression under vibration, in small quantities, until it was completely filled. After the gypsum had set (1 hour), the stone cast was separated from the impression, identified, and remained in a controlled environmental condition at 23±1°C, relative humidity between 40 and 60%, for 24±1 hour. After this period, the measurements of the stone casts were taken.
The stone casts were measured in a profile projector (VB300/P, Starrett, Athol, Massachussetts, USA) coupled to a digital measurement system (Quadra Check 200, Metronics, Mentor, Ohio, USA), with a accuracy of 0.001 mm, taking the measurements of the height (h) and diameter (d) of each specimen. Each stone cast was measured six times and an arithmetic mean was obtained for each sample. That way, the evaluation of the test specimen dimensional alteration (%) was verified by the formula:
ΔL=100x {(L1-L2)/L1}ΔL-dimensional alteration.
L1-stone cast dimension.
L2-dimension of the stainless steel pattern [Table/Fig-4].
Stainless steel pattern model used for obtaining the specimens.
Statistical Analysis
The data were submitted to statistical analysis using Bioestat 5.3 software (Mamirauá Institute, AM, Brazil, 2007). The Shapiro-Wilk test was applied to assess normal data distribution. Data were analysed by one-way ANOVA and Tukey’s test for comparisons of the means of the different groups, p<0.05 was considered statistically significant.
Results
The mean values for dimensional alteration obtained from the measurement of the test specimen diameters are shown in [Table/Fig-5]. Statistical analysis showed that groups I, II, and III had the highest means of dimensional alteration and did not differ among them. The group IV showed intermediate mean and differed statistically from the others, the group V differed statistically from the other groups and presented the lowest mean of dimensional alteration. Statistically significant differences were obtained on inter-group comparison using ANOVA (p=0.025).
Mean values and standard deviation (SD) of dimensional alteration (%) in diameter.
| Group | Mean (SD) |
|---|
| I- Control-impressions without any disinfection | -1.63 (0.48) a |
| II- Immersion in 2% glutaraldehyde | -1.61 (0.41) a |
| III- Immersion in 0.2% peracetic acid | -1.59 (0.58) a |
| IV- Nebulization with 2% glutaraldehyde | -0.89 (0.29) b |
| V- Nebulization with 0.2% peracetic acid | -0.27 (0.08) c |
Different letters in the same row mean statistically significant differences, p<0.05
The mean values for dimensional alteration verified from measurement of test specimen heights are shown in [Table/Fig-6]. Statistical analysis of these results demonstrated that groups I, II, III, and IV did not differ among them, while the group V showed the highest mean dimensional alteration. Statistically significant differences were obtained on inter-group comparison using ANOVA (p=0.022).
Mean values and standard deviation (SD) of dimensional alteration (%) in height.
| Group | Mean (SD) |
|---|
| I- Control-impressions without any disinfection | -1.39 (0.33) a |
| II- Immersion in 2% glutaraldehyde | -1.47 (0.24) a |
| III- Immersion in 0.2% peracetic acid | -1.48 (0.29) a |
| IV- Nebulization with 2% glutaraldehyde | -1.58 (0.26) a |
| V- Nebulization with 0.2% peracetic acid | -2.09 (0.54) b |
Different letters in the same row mean statistically significant differences, p<0.05
Discussion
Evaluation of the dimensional precision of stone casts obtained from impressions submitted to greatly differing processes of disinfection have been reported in scientific literature. The majority concluded that disinfection methods do not significantly alter the dimensional precision of stone casts [20]. However, no studies were found, which evaluated the dimensional precision of stone casts obtained from impressions disinfected with peracetic acid and the ultrasonic nebulization technique.
In this study, the null hypothesis was rejected, since the groups that were disinfected by nebulization presented different dimensional alteration when compared to control group. The results of the present study demonstrated that the impressions submitted to immersion, both in glutaraldehyde and in peracetic acid, as well as the control group, showed no statistically significant difference in their accuracy. Previous studies have found similarity among the experimental groups, immediately after using different disinfectant solutions with VPS, by immersion technique [20,21].
In the study carried out by Chen SY et al., a pattern model and a metal tray, similar to the ones used in this current study, were used for evaluation [14]. The accuracy differences, measured in stone casts from impressions made from several impression materials, were evaluated, however, not undergoing disinfection processes. In the above-mentioned study, the dimensional alteration values for the contraction percentage (%) of polyvinyl siloxane varied between 1.00±0.79–1.35±0.77.
The results found in this study showed that there was a contraction in all the evaluated stone casts compared to the stainless steel standards, which is a common finding in the literature [14,22]. Such results were observed as, during the polymerization reaction, the impression material retracted from the trays.
In the absence of a tray adhesive, there would be unrestricted polymerization shrinkage of the impression material, resulting in a cast that is smaller in diameter and height [21]. The tray adhesive was not used in this study because the trays had mechanically retentive features [Table/Fig-2]. In addition to this, the mechanical spatulation of the gypsum can be contributed to a lower setting expansion and, consequently, lower dimensional alteration of specimens.
The accuracy evaluation of dental VPS impressions submitted to ultrasonic nebulization techniques, using glutaraldehyde or peracetic acid solution, has not been reported in the literature yet. In this study, the analysis of the dimensional alterations of the test specimen’s diameter has shown that the ultrasonic nebulization, using glutaraldehyde solution, has shown statistically different values in relation to all the other experimental groups, presenting smaller dimensional alteration than the control group (I) and the immersion groups (II and III). On the other hand, the group of ultrasonic nebulization, using Sterilife™, has presented smaller dimensional alterations than all the groups. However, considering the height dimensional alteration of the samples, greater dimensional alterations than all the groups was found. Thus, ultrasonic nebulization, using peracetic acid, has shown the worst (2.09% for height) and the best results (0.27% for the diameter) for the dimensional changes in relation to the other groups. Therefore, it can be said that the interaction between the peracetic acid solution and ultrasonic nebulization processes interfered negatively in the accuracy of the models, causing shrinkage.
During ultrasonic nebulization, the concentration of the nebulized solution increases [11]. This is a caused by water evaporation during the aerosol output [23]. Corroborating with these statements, Steckel H et al., have found that changes in the droplet size, the surface tension, the viscosity and the saturated vapor pressure, during the ultrasonic nebulization process, can be caused by the temperature and the solution concentration in the nebulizer reservoir [24]. Such changes generally lead to an increase in the solutions’ concentration, which may additionally increase the molecule of the pharmacological solutions, which could, potentially, increase the adverse reactions to the nebulized liquid. Considering that the Sterilife® solution was used with ultrasonic nebulization, the droplet size may be changed, and the saturated vapor pressure increased, increasing the absorption effect of this solution in the polymer matrix of the VPS, causing a greater dimensional change to the stone mold. This situation, when transferred to the clinical practice, could act negatively in obtaining the stone mold used in the preparation of prosthetic crowns.
The selection of a disinfection technique should be established based on several criteria, besides the accuracy of the obtained stone casts, including the microbicidal capability of the disinfectant solution. In a study carried out by Mendonça MJ et al., the authors have evaluated the microbicidal capacity of 2% glutaraldehyde and Sterilife® solutions, applying disinfection methods: ultrasonic nebulization and immersion [13]. In this research study, the peracetic acid has shown microbicidal efficacy for both methods, and the glutaraldehyde has shown total microbicidal activity only for the impressions submitted to ultrasonic nebulization. Considering the results of the current laboratory study and the work by Mendonça MJ et al., it may be suggested that, in dental offices, the application of ultrasonic nebulization should be used, preferably, with glutaraldehyde solutions, whereas peracetic acid should be used with immersion techniques [13].
Limitation
The present study was an in vitro study; therefore it is necessary that future clinical studies should evaluate the difference of dimensional alteration when subjected to different disinfection techniques that influence the adaptation and indirect restorations.
Conclusion
It can be concluded that the immersion technique both in the glutaraldehyde and peracetic acid solutions did not interfere in the accuracy of the stone casts obtained, in comparison to the control group. The ultrasonic nebulization method associated with 2% glutaraldehyde solution did not differ significantly for height and even presented better values of dimensional accuracy of diameter compared to the control group. The ultrasonic nebulization method associated with 2% peracetic acid solution presented the worst values for height and better dimensional accuracy values for the diameter.
Different letters in the same row mean statistically significant differences, p<0.05
Different letters in the same row mean statistically significant differences, p<0.05