High-risk HPV infection caused by cervical intra-epithelial neoplastic disorders is a long-term, reversible pre-cancerous lesion and early intervention can effectively prevent cervical cancer [6]. Accordingly, a prophylactic vaccine was developed, which is now utilised worldwide; however, many ongoing studies are focusing on the development of a therapeutic vaccine [2]. Vaccination of successive cohorts of girls has the potential to reduce the average lifetime risk of developing cervical abnormalities and cervical cancer [7]. The incidence of cervical cancer is expected to decrease rapidly in the future, as a result of HPV vaccination [2]. It is essential to evaluate the prevalence of HPV types in different geographical regions before the large-scale implementation of prophylactic HPV vaccination, perform HPV testing in clinical practice, and monitor the impact of these procedures on cervical cancer control [8].
The purpose of this study was to evaluate the incidence of HPV infection in Dankook University hospital in Cheonan province in Korea during the past 5 years (2013-2018) and its changes over time. The results of the present analysis is expected to provide basic information essential to the development of vaccination strategies for the prevention of single and multiple HPV infections and for use in public women health programs based on HPV testing.
Materials and Methods
This retrospective study was approved by the Institutional Review Board (IRB) of Dankook University (IRB Approval No: 2016-08-009), conformed to the tenets of the Declaration of Helsinki, and was conducted at Dankook University. A total of 7,874 consecutive cervical swab specimens were collected for HPV test during the study period at Dankook University hospital and test results were retrospectively collected.
Sample Collection
A total of 7,874 cervical swab specimens were obtained from women aged 21-81 years who underwent a check-up at the Health Improvement Dankook University Hospital in Cheonan, Korea, and were referred for HPV genotyping between December 2013 and May 2018. The cervical swabs were collected using a cervical brush and specimen transport medium (Digene, Gaithersburg, MD, USA).
HPV DNA Detection and Quantification by Multiplex Real-Time PCR
The DNA of clinical specimens was extracted from 350 μL of cervical brush specimens using the QIAcube platform (Qiagen, Hilden, Germany). The extracted nucleic acids were then amplified and HPV detection and genotyping were performed using the AnyplexTM II HPV28 Detection Kit (Seegene, Seoul, Korea) and the CFX96 real-time thermocycler (Bio-Rad, Hercules, CA, USA) according to the manufacturer ’s instructions.
HPV Type Classification
The 28 HPV types were classified into three groups: High-Risk (HR), probably or Possibly High-Risk (PHR), and Low-Risk (LR). The HR group included HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66; PHR included Types 26, 53, 68, 69, 70, 73, and 82; and LR included Types 6, 11, 40, 42, 43, 44, 54, and 61 [8-10]. Semi-quantitative levels of these types were determined as the number of copies per reaction of each detected HPV type and categorised as follows: “+,” <102 copies/reaction; “++,” ≥102 but <105 copies/reaction; and “+++,” ≥105 copies/reaction.
Statistical Analysis
HPV data were analysed using R (version 3.3.3, Comprehensive R Archive Network; https://www.r-project.org), and the results are presented as averages or ranges. The chi-square test was used to analyse categorical data. The prevalence and 95% confidence intervals were calculated for the overall HPV genotypes and each individual genotype. All comparisons were evaluated by chi-square tests. p-values <0.05 were considered to indicate statistical significance.
Results
The total number of HPV-positive specimens was 1,457; the number of viruses detected was 2,131; positive samples showed 1.46 viruses per sample; and the positive HPV detection rate was 18.5%. The average age of all the patients was 38.6±8.55 years, and that of the HPV-positive patients was 36.4±9.41 years (21.7-80.2 years), [Table/Fig-1].
Number of samples, virus detection rate, and average age of patients during the study period.
| Variables | Number | Ratio | Average age of patient (years) |
|---|
| Sample | 7,874 | 100.0% | 38.6±8.55 |
| Positive | 1,457 | 18.5% | 36.4±9.41 |
| Negative | 6,417 | 81.5% | - |
| Virus | 2,131 | 1.46/positive sample | - |
Virus Type
The most commonly detected virus types were 52 (n=223), 68 (n=185), and 39 (n=154) [Table/Fig-2a]. Type 11 was most frequently categorised as +++ (53.3%), followed by Types 31 (41.3%) and 35 (40.5%). The lowest detection rate of +++ was observed for Type 39 (7.1%), followed by Types 54 (10.6%) and 45 (14.3%) [Table/Fig-2b]. A young average age was observed for HPV-positive patients infected with Type 87 (30.9 years), and an older age was observed for patients infected with Type 69 (48.7 years) [Table/Fig-3a]. The average age of patients with respect to infection strength was 36.6 years for +, 35.0 years for ++, and 33.5 years for +++ [Table/Fig-3b].
Proportion of infected samples and semi-quantitative levels of HPV types. a) Number of infections by HPV type. The blue bar represents low-risk; orange bar, high-risk; and grey bar, a probably or possibly high-risk infection. Black numbers indicate the most common HPV types, and red numbers indicate the least common types. b) Ratio of semi-quantitative levels of the HPV types. Bold numbers indicate the types that account for the largest percentage of each semi-quantitative level.
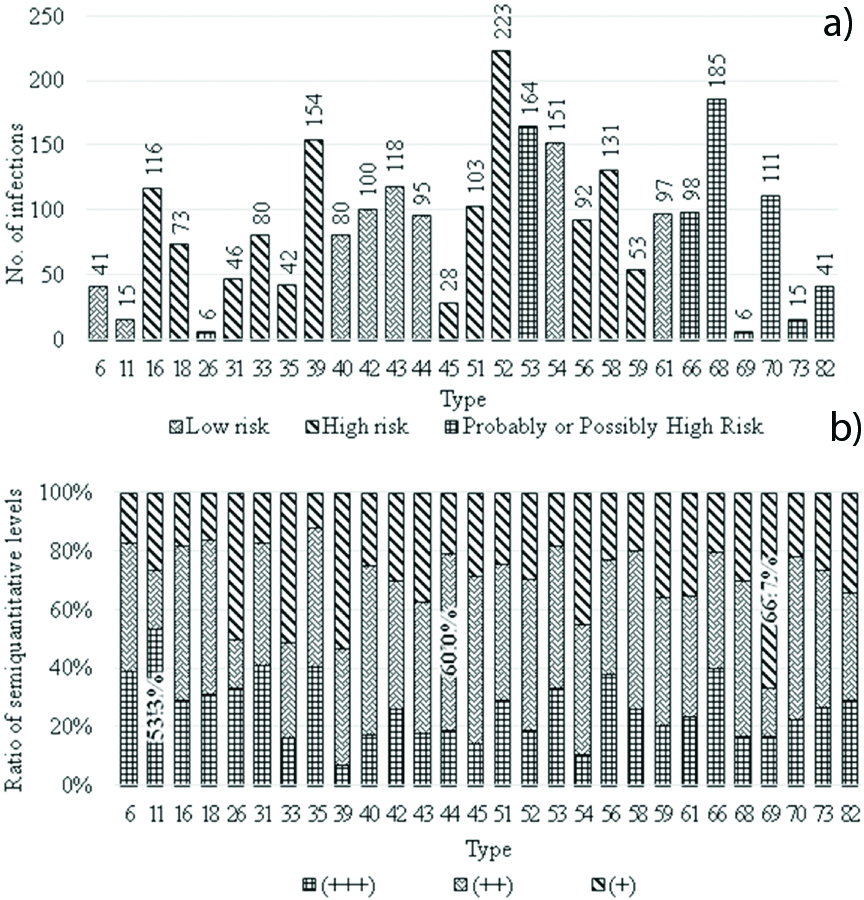
Average age of patients according to HPV type and semi-quantitative levels of the types. a) Average age by HPV type. Blue bar represents low-risk; orange bar, high-risk; and grey bar, probably or possibly high-risk infection. b) Average age by semi-quantitative levels of the types. The stronger the semi-quantitative levels, the lower the average age of the patient.
Infection Type
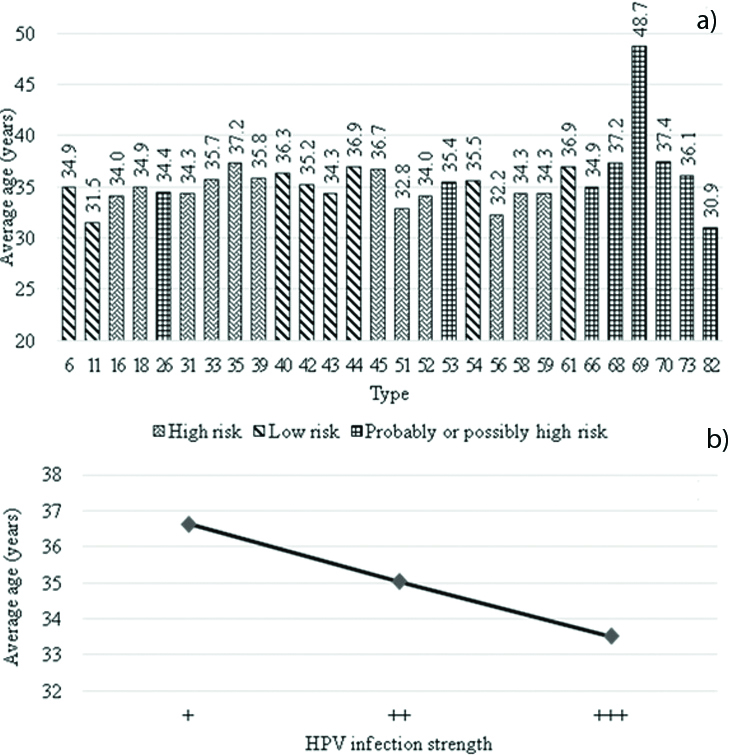
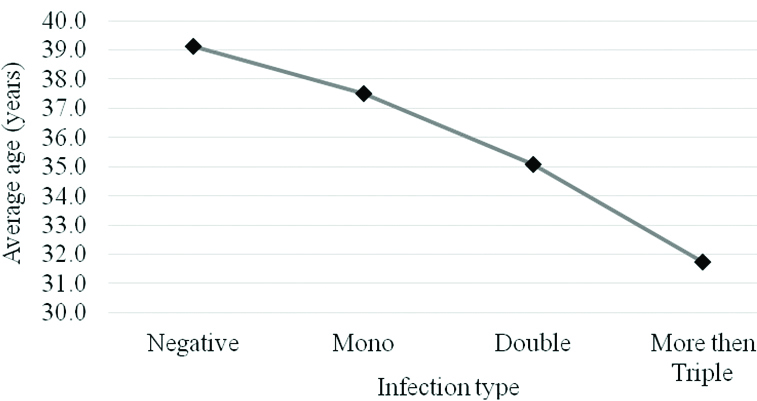
Of the 1,457 positive specimens, 1,025 (70.4%) were single infections, 284 (19.5%) were double infections, and 148 (10.2%) were multiple infections with three or more infections. The ratio of multiple to single infections for various age groups was 49.7% for patients in their twenties and 31.0% for those in their sixties [Table/Fig-4]. The average age of patients with a single infection was 37.5 years; that for patients with a double infection was 35.1 years; and that for patients with triple or more infections was 31.7 years [Table/Fig-5].
Rate of multiple HPV infections categorised by age group.
| Age group (years) | Total | Single infection | Multiple infection | Double infection | At least triple infection |
|---|
| 20-29 | 362 | 182 (50.3%) | 180 (49.7%) | 101 (27.9%) | 79 (21.8%) |
| 30-39 | 597 | 462 (77.4%) | 135 (22.6%) | 92 (15.4%) | 43 (7.2%) |
| 40-49 | 394 | 306 (77.7%) | 88 (22.3%) | 74 (18.8%) | 14 (3.5%) |
| 50-59 | 75 | 55 (73.3%) | 20 (26.7%) | 11 (14.7%) | 9 (12.0%) |
| 60-99 | 29 | 20 (69.0%) | 9 (31.0%) | 6 (20.7%) | 3 (10.3%) |
| Total | 1,457 | 1,025 (70.4%) | 432 (29.6%) | 284 (19.5%) | 148 (10.2%) |
Average age of patients according to negative and multiple infections by HPV types. The greater the number of duplicate infections, the lower the average age of the patient.
Year
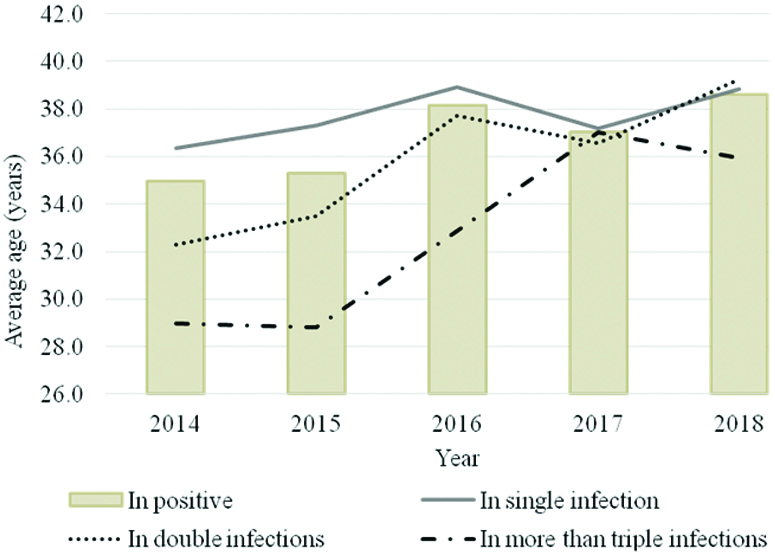
When infection rates were analysed by year, 2013 was excluded owing to the low sample size (n=14). The proportions of HPV-positive specimens per year were as follows: 20.7% in 2018, 19.2% in 2015, and 18.8% in 2016 [Table/Fig-6]. The average age was the highest at 38.6 years in 2018 and the lowest at 35.0 years in 2014 [Table/Fig-7].
Rate of multiple HPV infections over time.
| Year | Total | Positive (per submitted) | Single infection | Multiple infection | Double infection | At least triple infection |
|---|
| 2014 | 2,106 | 389 (18.5%) | 282 (13.4%) | 107 (5.1%) | 73 (3.5%) | 34 (1.6%) |
| 2015 | 1,741 | 334 (19.2%) | 215 (12.3%) | 119 (6.8%) | 73 (4.2%) | 46 (2.6%) |
| 2016 | 1,770 | 333 (18.8%) | 244 (13.8%) | 89 (5.0%) | 58 (3.3%) | 31 (1.8%) |
| 2017 | 1,726 | 291 (16.9%) | 209 (12.1%) | 82 (4.8%) | 58 (3.4%) | 24 (1.4%) |
| 2018 | 463 | 96 (20.7%) | 65 (14.0%) | 31 (6.7%) | 20 (4.3%) | 11 (2.4%) |
Average age of patients according to the year of each HPV infection type. The yellow bar shows the age for all HPV-positive patients. The blue line shows the age of patients with single infections; orange line, the age of patients with double infections; and grey line, the age of patients with at least three infections.
Discussion
This study found a high incidence of HPV infection and high frequency of multiple infections in the “Cheonan province”, indicating the need for intensive management in young women. Cervical cancer is common, but it has a long-term pre-cancerous stage, so mortality may be reduced if appropriate screening and vaccination are used [11-14]. The type of vaccine should be differentiated in consideration of the type and age of the prevalent HPV because HPV types and ages vary among geographical regions.
HPV vaccines can also help prevent infection with HPV. The HPV vaccination rate in Korea was 28.7% for 19-26 years old, 15.9% for 27-39 years old, and 4.6% for 40-59 years old, with an average of 12.6% in 2013. This is a very low figure compared to those in the US, UK and Australia [15].
The HPV detection rate is high in Africa, with a lower prevalence in North America and Europe. In 2015, the incidence rates of HPV infection were 15.18% in Europe, 16.69% in North America, and 17.26% in Oceania and the global average was 18.93% [16]. In this study, the HPV positivity rate was 18.5%, similar to the Latin American average of 18.63% and the global average [16].
The most common HPV types detected were Type 52 (14.2%), 81 (11.0%), and 58 (8.3%) in China [6]; 16 (30.1%), 52 (14.3%), and 51 (11.6%) in Spain [5]; 16 (38.6%), 18 (14.7%), and 6 (11.9%) in Iran [17]; and 16 (62.5%), 6 (8.9%), and 51 (7.1%) in Brazil [18]. HPV16 is the most prevalent type worldwide, at 13.7%, followed by types 31(11.8%) and 33 (8.4%) [16]; however, in this study, Type 52 (9.1%) was the most common, followed by Type 68 (7.5%) and 53 (6.7%). The average age of infected patients differed according to the HPV type. The youngest age for infection was observed for Type 87 (31 years), and the oldest age was observed for Type 69 (49 years). However, the number of subjects was small (Type 69, n=6), and the difference was not statistically significant. There was a significant difference in age with respect to copy number (p=0.005), with averages of 36.6 years for +, 35.0 years for ++, and 33.5 years for +++.
In this study, the proportion of individuals with multiple HPV infections was 29.6%. This is higher than the multiple HPV infection rate in Croatia (10%) [8], lower than the rate in China (42.9-49.2%) [6, 19], and similar to that in Brazil (26.1%) [20]. The average age for the multiple infections was lower when the patients were infected with more virus types (p=0.001). In addition, when infections were analysed according to age group, the rate of overlapping infection was the highest (49.7%) for individuals in their twenties (p=0.016). With respect to year, the average age of HPV-infected patients increased gradually, but the difference in age among the years was not statistically significant (p=0.054). However, the increasing trend in the average age of individuals with multiple infections was significant (p=0.028). From 2014 to 2018, there were no changes in the incidence of HPV infection, single infections, or multiple infections (p>0.05).
Limitation
First, it was limited to a single area, Cheonan, in Korea and to a relatively short period of five years. Since it was a retrospective study, additional data could not be obtained after the analyses. Nevertheless, we believe that meaningful results were obtained.
Conclusion
The incidence of HPV infection and the average age of the infected patients were similar to the global averages. While Type 16 and 18 are frequently detected internationally, Type 52 was particularly unique in this study. The high incidence of HPV infection and high frequency of multiple infections observed emphasise the need for intensive management in young women. The results of this study will contribute to the prevention, treatment, and long-term care of patients with HPV infection and will facilitate policy-making and public health programs.