The time-honored technique of histopathology involving tissue fixation and processing, as developed in the 19th century, is being practiced till date [1]. This conventional method, which has been in vogue for over a century, is labor intensive and slow, taking 16-20 hours. This results in considerable delay in biopsy reports.
An early diagnosis of tissue biopsies would be highly desirable as it would facilitate early decision making and initiation of treatment, especially for patients with neoplastic diseases. It would also reduce the duration, and thereby the cost of hospital stay, improving overall patient satisfaction.
Some authors have described a modification to the conventional method of processing, wherein tissues can be processed within minutes using a microwave. They have shown that tissues processed in a microwave are equal to or even superior in quality to those prepared by the conventional method when stained with not only Haematoxylin and Eosin (H&E) but also special histochemical stains and immunostains [2-14]. While some studies have assessed quality of H&E staining of microwave processed tissues, others have focused on special histochemical stains and still others, on immunohistochemistry. Very few studies have addressed the staining characteristics of all three types of stains on same tissues [5,11].
This study was undertaken to compare microwave-assisted tissue processing with conventional tissue processing, with the aim of reaffirming its usefulness in rendering a diagnosis based on routine and special stains, in the light of its markedly reduced turnaround time.
Materials and Methods
The study was conducted at Base Hospital, Delhi, a large tertiary care hospital. It was a prospective study spanning a period of one year from November 2017 to October 2018. A total of 40 pairs of different types of tissues were studied, after obtaining ethical clearance. Specimens were received in 10% formalin. From each specimen, pairs of 1.0×1.0×0.5 cm slices were taken. One of each pair of tissue formed the test group and was processed using microwave irradiation. These tissues were enclosed in microwave safe plastic cassettes and placed in microwave safe plastic jars containing different reagents. Initially the procedure was standardised using 10, 20 and 30 seconds of irradiation at full power (700W) followed by 2, 5 and 10 minutes of incubation for each step until good results were achieved. After optimisation of timing, all test samples were irradiated at full power and then incubated in switched off mode in a domestic microwave for specified durations [Table/Fig-1]. The tissues were then impregnated with wax at 56°C for 15 minutes and embedded in wax to form tissue blocks.
Comparison of protocols for microwave-assisted processing and conventional processing.
| Reagent | Time required for microwave-assisted method | Time required for conventional method |
|---|
| Irradiation | Incubation | |
|---|
| 10% formalin | 30 sec | 5 min | 4 hr 30 min |
| 50% ethyl alcohol | - | - | 1 hr |
| 70% ethyl alcohol | 20 sec | 2 min | 1 hr |
| 90% ethyl alcohol | 15 sec | 2 min | 1 hr |
| 95% ethyl alcohol | - | - | 1 hr |
| 99% ethyl alcohol | 10 sec | 2 min | 1 hr 20 min |
| Acetone | 10 sec | 2 min | - |
| Xylene | 60 sec | 5 min | 2 hr 40 min |
| Wax (56°C) | - | 15 min | 4 hr 30 min |
| Total time taken | 35 min 25 sec | 17 hr |
The other tissue from each pair formed the control group and was processed by the conventional method in an automated tissue processor (Unimeditrek VTP-300) [Table/Fig-1]. The tissues were then embedded in wax to make tissue blocks.
From both, test and control groups of tissue blocks, 3-4 micrometer sections were cut. All sections were stained with H&E. In addition, certain special histochemical stains were performed on 13 pairs of tissues. Of these, 10 pairs of tissues of the gastrointestinal tract were stained with Periodic Acid Schiff (PAS) stain and Masson’s Trichrome (MT) stain and two pairs of skin biopsies and one pair of tissue from ovarian dermoid cyst were stained with Zeihl Neelsen (ZN) stain.
Immunohistochemistry (IHC) was performed on 32 pairs of test and control groups of tissues. Of these, 16 tissue pairs with epithelia were stained with Pan-Cytokeratin (Pan-CK), four lymphoid tissue pairs were stained with CD45 and CD5 and 12 tissue pairs containing nerve were stained with S100 antibodies. The respective primary antibodies were applied to the tissue sections for 60 minutes, followed by biotin labelled secondary antibody. Biotinylated-avidin coupled horseradish peroxidase was then applied. Following this, diaminobenzidine was added until the development of brown colour was observed.
The tissue sections were counterstained with haematoxylin.
The tissue sections were then allotted computer generated random numbers with no indication of the group to which they belonged and were evaluated by a senior pathologist. The sections stained with H&E were evaluated for seven morphological characteristics as used by Mathai AM et al., Tripathy M et al., and Titford ME et al., in their studies [3,4,15]. The seven parameters, namely, overall morphology, overall staining, cellular outline, cytoplasmic detail, nuclear detail, Red Blood Cell (RBC) integrity and appearance of inflammatory cells, were scored on a scale of 1 to 4 (1-poor, 2-fair, 3-good, 4-excellent) [3]. Overall grading was obtained by adding the scores of each parameter; a score ranging between 7 and 13 was graded as ‘poor’, between 14 and 20 as ‘fair’, between 21 and 27 as ‘good’ and a score of 28 was graded as ‘excellent’. Statistical analysis was performed using SPSS 21.0. The pathologist also added a comment on whether the tissue section was optimal for reporting.
All sections stained with special histochemical stains and immunohistochemical stains were evaluated for both, positive staining, and lack of it, in positive and negative control areas, respectively, present within each tissue section. An additional comment was added on whether the section was optimal for reporting.
Results
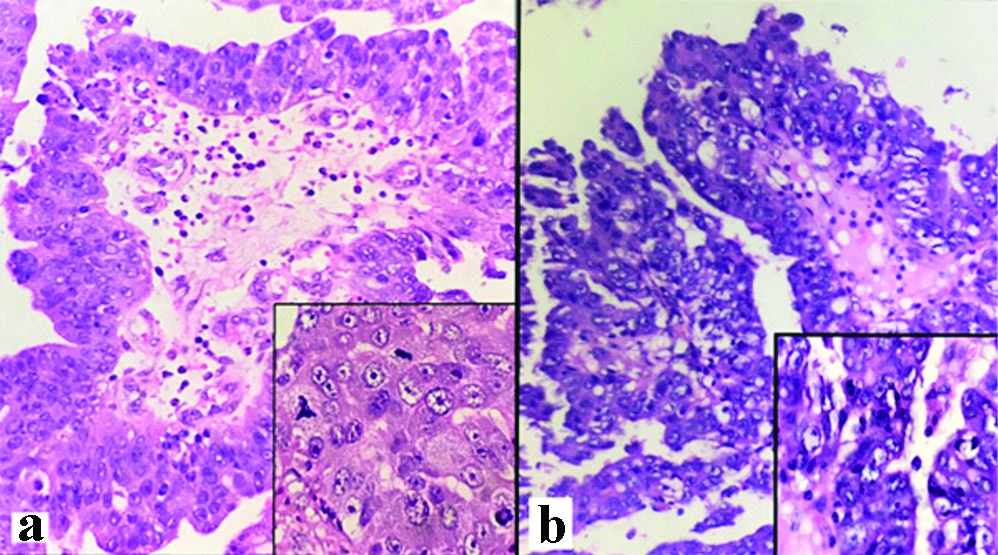
The quality of all H&E stained tissue sections processed by both methods was either good or excellent. Of the 40 pairs, 37 (92.5%) pairs were found to have the same grade in test and control groups; of the remaining three pairs, two (5%) pairs were found to be of excellent quality by microwave-assisted processing and good quality by conventional processing, while in the remaining one (2.5%) pair, the conventional method showed an excellent result while the microwave-assisted method showed a good result [Table/Fig-2]. The strength of agreement between the results obtained by the two methods was very good (Kappa coefficient: 0.806). The marginal difference in the quality of sections by the two methods did not affect evaluation of the histomorphology and rendering of diagnosis. All sections of both groups showed optimal preservation of architecture and crisp nuclear and cytoplasmic staining [Table/Fig-3]. Among both groups, nuclear chromatin and nucleoli were discernible, cytoplasm showed good staining and cell outlines were well delineated. Different types of inflammatory cells were easily identified. Twenty-seven sections of the test group and 26 sections of the control group showed mild distortion in the shape of RBCs; this, however, did not interfere with the diagnoses. No lysis of RBCs was noted in any section of either group.
Correlation of quality of H&E stained sections obtained by the two methods of tissue processing.
| Microwave-assisted | Total |
|---|
| Excellent | Good |
|---|
| Conventional | Excellent | 9 | 1 | 10 |
| Good | 2 | 28 | 30 |
| Total | | 11 | 29 | 40 |
Papillary serous carcinoma ovary; H&E, x400 (Inset x1000). a: Microwave-assisted processing. b: Conventional processing.
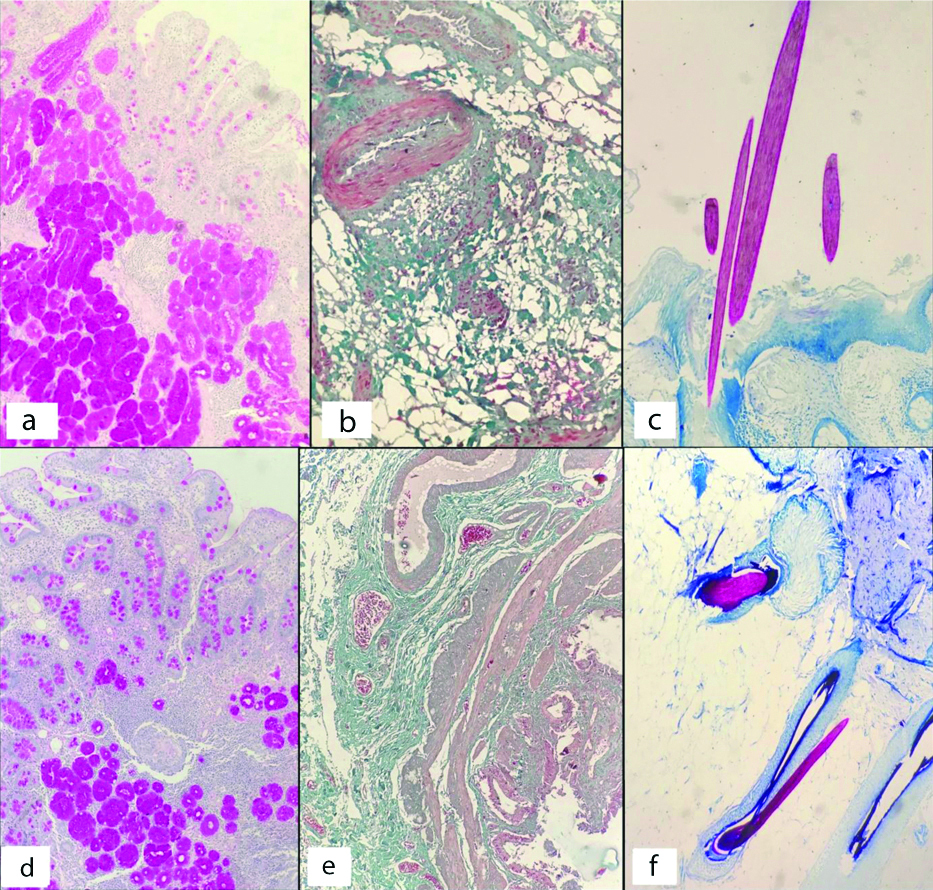
All sections of test and control groups stained with special histochemical stains showed good results [Table/Fig-4]. Both sets of tissues showed selective staining of epithelial mucin by PAS stain and good differentiation of RBCs, smooth muscle and fibrous tissue by MT stain. ZN stain of all three pairs of skin tissue of both groups showed good staining of acid-fast hair shafts.
a to c– Special histochemical stains by conventional processing, x100. a: PAS staining of epithelial mucin in gastrointestinal tract. b: MT staining of RBCs, smooth muscle and fibrous tissue in gall bladder. c: ZN staining of acid-fast hair shafts. d to f– Special histochemical stains by microwave-assisted processing, x100. d: PAS staining of epithelial mucin in gastrointestinal tract. e: MT staining of RBCs, smooth muscle and fibrous tissue in gall bladder. f: ZN staining of acid-fast hair shafts.
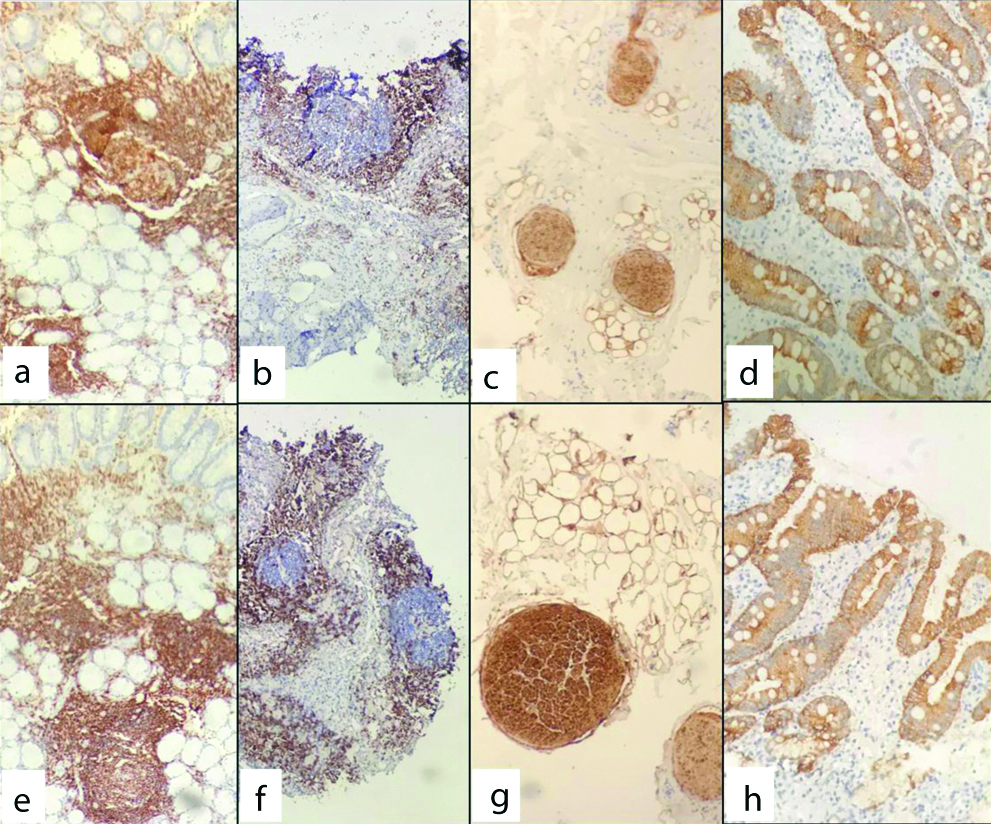
IHC on tissue sections of both groups showed crisp, selective staining [Table/Fig-5]. Pan-CK showed positive staining of epithelia and negative staining of subepithelial connective tissue; CD45 showed positive staining of all lymphoid cells and negative staining of epithelium and subepithelial connective tissue; CD5 showed selective staining of T cells located in the paracortical region of reactive lymphoid tissue; S-100 showed selective staining of nerves.
a to d– Immunohistochemistry by conventional processing, x100. a: CD45 in lymphocytes. b: CD5 in T lymphocytes. c: S-100 in nerves. d: Pan-CK in epithelium. e to h– Immunohistochemistry by microwave-assisted processing, x100. e: CD45 in lymphocytes. f: CD5 in T lymphocytes. g: S-100 in nerves. h: Pan-CK in epithelium.
Discussion
The conventional method of tissue processing has been in practice for over a century without much change. Each step of the process is time consuming and contributes to the long turnaround time of biopsy reports, causing undue delay between surgical biopsy and diagnosis. Hence, the necessity to explore ways and means to shorten the processing time was the need of the hour.
Heat causes partial denaturation of protein. This forms the basis for tissue fixation. Microwaves are electromagnetic waves which excite polar molecules in a medium, causing them to oscillate and collide with adjacent molecules, producing heat. In 1970, Mayers recognised the potential application of microwave energy and successfully fixed the tissue with a microwave generator [16]. He demonstrated that microwave energy penetrates rapidly, overcoming poor heat conduction through tissue, raising its temperature and fixing it in a shorter time [16]. Heating by microwave is uniform throughout the tissue as opposed to simple heating which is non-uniform. When tissues are placed in a fixative solution in a microwave, the heat thus produced also reduces the viscosity of the fixative and processing fluids, causing the reagents to diffuse into the tissue at a faster rate.
Some authors have used phosphate buffered saline as a fixative in microwave and have reported useful but suboptimal results due to some tissue loss; others have used formalin with better results [3,4]. To ensure adequate fixation of tissues for microwave-assisted processing, Singla K et al., and Bhuvanamha DR et al., preceded microwave fixation by conventional fixation with 10% formalin for 12 to 24 hours [7,10]. However, our study has shown that good fixation can be achieved with 10% formalin after 30 seconds of microwave irradiation followed by five minutes of incubation. No prior prolonged fixation in formalin was necessary. Mathai AM et al., too have achieved adequate fixation with 20 minutes of irradiation with 10% formalin without prior prolonged fixation [3]. These findings suggest that prolonged conventional fixation need not precede microwave fixation.
The time required for tissue fixation also depends on the thickness of tissue. Our study included tissue sections of size 1.0×1.0×0.5 cm to ensure rapid and uniform fixation. Sivadas P et al., studied microwave-assisted processing of tissue of greater, lesser and same size as in our study; they found optimal results with tissues of size similar to ours [8]. Smaller biopsies would require lesser time for fixation and therefore, would require standardisation based on their size.
Following fixation, microwave also hastens each step of tissue processing such as dehydration, clearing and impregnation by causing the respective reagents to penetrate rapidly. This brings down the duration of tissue processing from several hours to minutes. The complete tissue processing technique using a microwave was introduced by Boon ME et al., [9]. Since then, the technique has been tested and endorsed by several authors who have established that microwave irradiation can reduce the tissue processing time markedly [2-11]. We achieved good results with 29 minutes and 55 seconds of processing after initial fixation with formalin for five minutes and 30 seconds.
Our study has shown that tissues processed by microwave produce sections that are comparable with tissues processed by the conventional method, with only a marginal difference in quality. This difference affected neither the histomorphological evaluation nor the diagnoses. Other studies also have shown similar results using the two methods of tissue processing [2-11].
Our study also demonstrated that the sections obtained by microwave processing yielded optimal results with special histochemical stains, as did sections obtained by the conventional method. Furthermore, both sets of tissue sections showed crisp and specific staining with commonly used immunohistochemical stains. Other authors, too, have obtained good results with special histochemical stains and IHC on tissues processed by microwave [12-14].
Our findings prove that this less explored method of microwave processing has great potential in providing early diagnosis of tissue biopsies which can benefit patients in several ways. One such benefit is its scope as a method of intraoperative tissue diagnosis. Availability of quick intraoperative tissue diagnosis is of great value in clinical situations like confirming metastatic disease during staging laparoscopies/laparotomies, diagnosing malignancy intraoperatively in suspected cases so that oncological surgery can be done at the same time, and determining intraoperative negative margins to aid in oncological clearance during surgery. This vital information, if provided timely, can avoid inadequate and inappropriate surgeries. It can also prevent the need for a revision surgery later once the tissue diagnosis is available by the conventional method, thereby avoiding additional burden on the hospital and adverse psychological effect on the patient. Traditionally, intraoperative tissue diagnosis is provided by frozen section because of its short turnaround time; however, the technique has a few problems like freezing artefacts, poor quality brain and adipose tissue sections, relatively poor staining and inferior cell morphology [17].
In hospitals that do not have a cryostat, which is a prerequisite for frozen section, intraoperative diagnosis is provided by scrape and crush smears of tissue, but the sensitivity of these methods is less than that of tissue sections [18]. This is because they rely on cytomorphology instead of histomorphology. In such situations, microwave processing can serve as an excellent alternative, providing timely and reliable information from tissue sections which are comparable in quality to those obtained by the time honoured, albeit time consuming, conventional method of tissue processing.
Another significant impact that microwave assisted tissue processing can have is in reducing the turnaround time for routine tissue diagnosis by providing reports on the same day. The tissue processed in this manner, by lending itself not just to routine evaluation but also to ancillary tests like special histochemical stains and IHC, is comparable to conventional processing in every way. It would aid greatly in early decision making and reduction of hospital stay and would also ensure less frequent patient visits to the hospital.
Limitation and Future Recommendations
The limitations of our study stem largely from the use of a domestic microwave. The limited throughput owing to the small size of the microwave, release of noxious fumes due to heating of reagents and labour-intensive manual processing make it prohibitive for processing routine biopsies on a regular basis. To overcome these problems large, laboratory grade microwave tissue processors with built in exhaust fans to extract toxic vapours and automated control of power, temperature and processing time would be recommended.
Conclusion
Microwave assisted tissue processing can significantly reduce turnaround time for biopsies without compromising the quality of tissue sections. Tissues processed by microwave, by being amenable to H&E and various ancillary tests like special histochemical stains and IHC, can provide a lot of information in a remarkably short time. This can pave the way for providing same day biopsy reports and also have a role in intraoperative diagnosis.