The concept of regional anaesthesia has been around for almost a century now; the renewed enthusiasm can be attributed to improved patient outcomes, both peri-operative and long-term. Several studies have established the superiority of neuraxial techniques over General Anaesthesia (GA) by demonstrating reduced surgical stress, better medical and economic outcomes, and fewer respiratory complications, infections (by affecting immune response) and need for ICU care [1-3].
Neonates have the neurological mechanisms at birth to experience painful stimuli; the nociceptive receptors are functional as early as 25 weeks’ gestation [4]. The morbidity of inadequately treated pain is significant and may result in physiological instability, altered mental development and inappropriate stress response. The repair of oesophageal atresia by thoracotomy (or thoracoscopy) is one of the most common neonatal surgical emergencies. Peri-operative pain management in these patients is challenging due to fear of respiratory depression post-opioid administration.
The use of epidurally administered analgesics is now a well-established technique. The use of lipophilic steroids such as fentanyl is preferred over their hydrophilic counterparts which are long-lasting and portend a higher risk of sedation and respiratory depression due to more extensive ‘intra-spinal1’ spread. Their mechanism of action is debated between segmental analgesia by local action on dermatome specific nerve roots and systemic analgesia post-vascular absorption and supra-spinal site of action [5-7]. However, if the supra-spinal site of action is a reality, epidural fentanyl should not score superior to intravenous (systemic) administration.
With this background, the authors conducted this study to compare the relative efficacy of epidural analgesia vis-à-vis intra-venous analgesia in patients of oesophaeal atresia undergoing thoracotomy for primary repair of oesophageal atresia. The outcome measures under study included heart-rate (baseline or pre-incision and post-incision), need for fentanyl boluses (number of boluses required and total fentanyl consumption), temperature (at end of surgery), non-invasive blood pressure (before and after surgery), extubation on-table after surgery, need for post-operative ventilation, pain (scores) and complications of epidural administration.
Materials and Methods
This was a randomised controlled trial conducted in the Department of Paediatric Surgery at a tertiary care centre on 60 consecutive neonates of oesophageal atresia undergoing surgery by thoracotomy under care of single, senior paediatric surgeon from July 2016-June 2018 (inclusion criteria). All the research in this study were performed in accordance to the Declaration of Helsinki (1964).
Patients with gestational age <30 weeks, birth weight <2.0 kg, septicaemia, congenital cyanotic heart disease, need for ventilation prior to surgery, and those with associated gastrointestinal anomalies other than low anorectal malformation were excluded from the study (exclusion criteria).
Work-up included complete blood count, serum electrolytes, renal function tests, coagulation profile, radiography of chest (with a red rubber catheter in upper pouch), ultrasonography of kidney, ureter, bladder region and screening echocardiography.
Eligible neonates were assessed clinically and data pertaining to their age, sex, birth weight, maturity, presence of pneumonia and other associated anomalies were recorded. Participants were randomised into two groups (A and B) based on a computer-generated random number program (allocation ratio 1:1). This was a double-blinded study. Herein, the participant in the study (the neonate, the parents and relatives in this case) and surgical team were blinded to the study group of the participant. The operating surgeon was not allowed to enter the theatre while the patient was being prepared for surgery. For those patients who were not given epidural, a dummy tube was fixed in position so that all patients look alike to the surgeon. The investigator assessing for pain wasn’t aware of the group of the patient. However, the anaesthetist team were aware of the allocation of the case to specific groups.
All children were taken up for elective, right postero-lateral thoracotomy and repair under general anaesthesia. Intravenous access was established, and the neonates were pre-oxygenated with 100% oxygen for 3-5 minutes. Monitoring with pulse oximetry, non-invasive blood pressure, heart rate and end-tidal carbon di-oxide were set-up. Injection Atropine @0.01 mg/kg intravenous was given as premedication followed by induction with sodium thiopentone @4-6 mg/kg and succinylcholine @1 mg/kg. Endo-tracheal intubation was performed under direct laryngoscopy with Miller’s laryngoscope blade (endotracheal tube size 2.5-3.5 mm internal diameter); the tip of the tube was positioned distal to the tracheao-esophageal fistula. The presence of air entry bilaterally was confirmed. Anaesthesia was maintained with nitrous oxide, oxygen and halothane targeting a Minimal Alveolar Concentration (MAC) of 0.8-1.0. Intermittent boluses of injection atracurium @0.1 mg/kg were used to provide adequate muscle relaxation.
Group A (n=30): With complete asepsis, a Tuohy epidural needle (18 G) was inserted via sacral hiatus into the epidural space (identified by loss of resistance to ‘saline-technique’). The anaesthetist performing has a significant experience in paediatric anaesthesia and pain medicine procedures. A 20 G thoracic epidural catheter was threaded through this needle and advanced intra-spinally to position the tip at the level of 6th-8th thoracic vertebra in the left lateral position.
A bolus dose of 0.25% bupivacaine @2 mg/kg with fentanyl @1 mcg/kg in 2 mL volume was administered. Surgical incision was allowed 10 minutes after the epidural bolus.
Group B (n=30): Baseline heart rate was recorded. Participants in this group were administered injection fentanyl @1 mcg/kg intravenously 10 minutes before incision for analgesia which was also repeated @0.5 mcg/kg whenever the heart rate increased by more than 20% from the baseline [Table/Fig-1].
At the end of surgery, inhalation anaesthetic agents were stopped, and residual neuromuscular blockade was reversed with neostigmine and glycopyrrolate.
Post-operative pain in both the groups was addressed with fentanyl administered whenever the pain score on the Neonatal Infant Pain Scale [8] was 3 or more. Injection paracetamol was administered intravenously @7.5 mg/kg to all before shifting out of the operation theatre and the same was continued six-hourly for post-operative pain relief, the assessor of pain was blinded to the study group of the participant.
The monitoring and pain assessment was done in first 24 hours, adequacy of respiration was assessed by respiratory rate and oxygen saturation.
No changes were made in the methodology after commencement of the trial.
Statistical Analysis
Data were represented as Mean (Range±SD). Independent sample t-test and Mann Whitney U test were used to compare the heart rate and total fentanyl consumption respectively between the two groups.
Results
The study was based on 60 neonates with oesophageal atresia with a mean birth weight of 2.78 kg (range 2-3.8 kg) of which 46 were born at term and 14 preterms (Preterm: Term=1:3.3). There were 35 boys and 25 girls (Male: Female=1.4:1) [Table/Fig-2].
Baseline characteristics of the patients.
| Demographic and baseline characteristics | Group A (n=30) | Group B (n=30) | p-value |
|---|
| Age in hours, mean±SD | 43.37±27.66 | 43.10±30.42 | 0.97a |
| Male gender, no. (%) | 17 (56.7) | 18 (62.1) | 0.67b |
| Weight in kg, mean±SD | 2.66±0.40 | 2.88±0.54 | 0.08a |
| Maturity, no. (%) |
| Premature | 6 (20.0) | 8 (26.7) | 0.14b |
| Mature | 0 (0.0) | 3 (10.0) |
| Full term | 24 (80.0) | 19 (63.3) |
| Chest complaints, no. (%) |
| Bilateral pneumonia | 5 (16.7) | 9 (30.0) | 0.39b |
| Left side pneumonia | 2 (6.7) | 4 (13.3) |
| Right side pneumonia | 5 (16.7) | 5 (16.7) |
| None | 18 (60.0) | 12 (40.0) |
| Associated anomalies, no. (%) |
| ARM | 6 (20.0) | 4 (13.4) | 0.59b |
| VSD | 1 (3.3) | 2 (6.9) |
| PDA | 1 (3.3) | 1 (3.3) |
| ARM+VSD | 7 (23.3) | 3 (10.3) |
| None | 15 (50.0) | 19 (65.5) |
aUnpaired t-test, bChi-square test
The demographic data when stratified between two groups (A and B) was comparable. Group A comprised of 30 neonates randomised to the epidural fentanyl group (mean birth weight 2.7 kg, range 2-3.5 kg). Group B comprised of 30 neonates randomised to the intra-venous fentanyl group (mean birth weight 2.9 kg, range 2-3.8 kg).
Mean age at surgery was 69.6 hours (range 8-264 hours). Of these 76.7% (n=46) neonates were operated within 72 hours of life. Mean age at surgery for boys and girls was 68.68±39.6 hours and 70.96±36.9 hours respectively. Mean age at surgery for neonates in Group A was 71.5 hours (range 8-194 hours). Mean age at surgery for neonates in Group B was 74.9 hours (range 10-206 hours). Statistically, the groups were comparable with regard to baseline demographic characteristics.
Heart rate: Mean baseline (Pre-incision) heart rate in the cohort was 177.5 per minute (range 140-201). The two groups were statistically comparable with regard to mean baseline heart rate {177.3 per minute (range 140-201) and 179.75 per minute (range 142-199) in Groups A and B respectively; p-value 0.13} [Table/Fig-3].
Comparison of study parameters.
| Group A (n=30) | Group B (n=30) | p-value |
|---|
| Temperature, no. (%) |
| Hyperthermic (>37.4°C) | 1 (3.3) | 0 (0.0) | 0.36a |
| Hypothermic (<36.0°C) | 8 (26.7) | 12 (40.0) |
| Normal (36.0-37.4°C) | 21 (70.0) | 18 (60.0) |
| Heart rate |
| Pre-incision | 177.1±14.1 | 171±11 | 0.13b |
| Post-incision | 179.4±10.9 | 186±9 | 0.01*b |
| Requirement of boluses of fentanyl in the intraoperative period, no. (%) | 2 (6.7) | 9 (30) | 0.02*a |
| Requirement of boluses of muscle relaxant, no. (%) | 11 (36.7) | 30 (100.0) | 0.0001*a |
| Immediate extubation at the end of surgery | 18 (60.0) | 0 (0.0) | 0.0001*a |
| Total analgesic in 24 hours (fentanyl), no. (%) | 14 (46.7) | 30 (100.0) | 0.0001*a |
| Requirement of post op ventilator beyond 6 hours, no. (%) | 2 (6.7) | 11 (36.7) | 0.005*a |
aChi-square test, bUnpaired t-test, cPaired t-test, *Significant
The post-incision heart rate (mean±SD) was 179.4±10.9 beats per minute in Group A and 192±9 beats per minute in Group B. The mean heart rate in Group B was higher than that of Group A; the difference between the groups was significant at a p-value of 0.01 (independent sample t-test). The rise in the heart rate in Group A with surgical incision was less than that observed in Group B.
Fentanyl boluses: Patients requiring intraoperative boluses of fentanyl were significantly higher in Group B (2 of 30 in Group A versus 9 of 30 patients in Group B, p=0.02, chi-square test). Total fentanyl consumption in Group A was significantly lower than Group B {median (IQR) 1.2 (0-2.5) mcg in Group A versus 7.75 (6-12) mcg in Group B; p<0.0001; Mann-Whitney U test}.
Temperature: There was no significant difference in the temperature at the end of surgery between the groups.
Non-invasive blood pressure: A significant decline in non-invasive blood pressure was observed in both the groups from before and after surgery (p=0.0001).
Extubation on OT-table post-surgery: In Group A, 60% (n=18) of the patients were extubated immediately after surgery in the operating room, whereas none of the patients (n=0) was extubated immediately in the Group B (p<0.0001).
Postoperative mechanical ventilation beyond 6 hours was significantly less in patients belonging to Group A (2 of 30 patients in Group A versus 11 of 30 patients in Group B); risk ratio (95% CI) was 0.26 (0.07-0.94) with p≤0.005 (chi-square test).
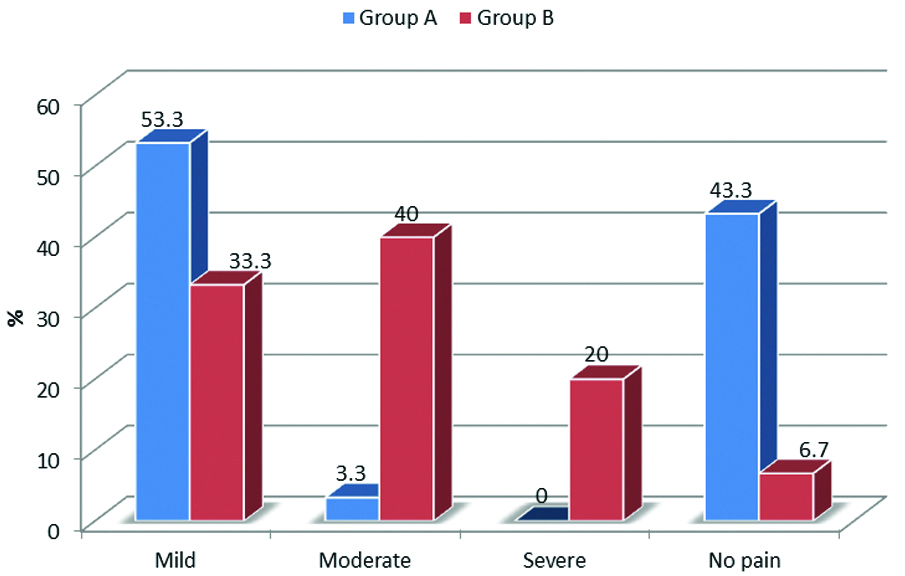
Pain: No pain was observed in n=13 (43.3%) patients from Group A and in n=2 (6.7%) from Group B (p=0.12, chi-square test). Mild pain was observed in n=16 (53.3%) patients from Group A and in n=10 (33.3%) from Group B (p=0.12, chi-square test) [Table/Fig-4]. Pain scores were at the time of extubation were significantly higher in Group B (p<0.001, Mann-Whitney U test).
Comparison of visual analog score at the time of extubation (chi-square p=0.0001, significant).
Complications of epidural: Inadvertent puncture of the dura occurred in five patients in Group A. The catheter was pulled-out accidentally in 1 patient.
Discussion
To the best of the present authors belief, this is the first study comparing the efficacy of pain relief with epidural fentanyl and bupivacaine with intravenous fentanyl in neonates undergoing surgery for oesophageal atresia. The authors have witnessed better pain-relief along with a significantly reduced need for postoperative mechanical ventilation in the neonates receiving epidural fentanyl and bupivacaine vis-à-vis intravenous fentanyl.
Pain is a sensation with strong emotional association [9]. The following traditional beliefs have been refuted :1) the neonatal response to painful stimuli are decorticate in nature; 2) neonates lack pain perception or localization and memories of painful experiences [10]; and 3) neonates have a high threshold to pain stimuli, which is a mechanism to adapt them to pain during labour [11].
It is known that the cortical and subcortical centres of pain perception are well developed in late gestation. Specific behavioural changes have been observed in neonates after circumcision which can disrupt their adaptation to the post-natal environment and other faculties [12-14]. Mounting evidence indicates that adult neurosis and psychosomatic illnesses may have their seeds in painful experiences of infancy or even neonatal period [15].
This study has witnessed that the rate of post-surgery on-table extubation was higher in Group A (n=18; 60% vis-à-vis n=0 in Group B). Even at six hours post-surgery, 2 (6.7%) patients of Group A and 11 (36.7%) patients of Group B were still ventilator-dependent.
Moreover, the need for supplemental analgesia during surgery was significantly less in Group A as compared to Group B in terms of both number of patients requiring fentanyl boluses (2 of 30 in Group A versus 9 of 30 patients in Group B) and total fentanyl requirements (14 of 30 in Group A versus 30 of 30 patients in Group B).
The risks of epidural opioids such as respiratory depression, suppression of cough reflex, urinary retention, sedation, nausea or vomiting) were obviated by addition of bupivacaine which enabled reduction in the total dose of epidural fentanyl.
The use of epidural analgesia may also reduce post-operative paralytic ileus in neonates which improves the surgical outcome [16].
Effective pain relief in intraoperative and post-operative period is very important during any surgical procedure. Herein, belies the importance of providing adequate pain relief to neonates; the better the pain relief, the lesser is the stress during surgery [17] and earlier is the post-operative recovery. Insufficient pain control during surgery may be catastrophic in view of short and long-term effects. There are reports stating that in paediatric surgical patients, three-fourth of the patients are administered insufficient post-operative analgesia [9,18].
Repair of oesophageal atresia is performed in left lateral position by a right postero-lateral thoracotomy. These factors coupled with the retraction of the right lung and the painful stimuli involved with the surgery result in ventilation-perfusion mis-match [19]. These patients are prone to hypoxia and exposed to heightened surgical stress which lead to augmented requirements of postoperative ventilation. Furthermore, it is known that post-thoracotomy pain can interfere with deep breathing and cough reflex which can lead to retention of airway secretions, bronchial plug formation and atelectasis [20].
Effective pain relief in intraoperative and postoperative period is very important to reduce the need of postoperative mechanical ventilation and to improve the surgical outcome. Various techniques like intercostal nerve blocks, intra-pleural block, epidural analgesia and intravenous analgesics have been described in literature to provide analgesia in these patients. Intercostal nerve block and intra-pleural analgesia may require large doses of local anaesthetics [21]. Higher plasma concentrations of such drugs may lead to drug toxicity in view of lower level of plasma protein alpha 1 acid glycoprotein and albumin in the neonates [22]. Gaeta RR et al., have demonstrated the superiority of analgesia provided by epidural (lumbar) hydromorphone over that with intra-pleural bupivacaine following thoracotomy [23].
The use of intravenous opioids for post-operative analgesia has its own complications like respiratory depression, nausea and vomiting. Rocca GD, [24] has compared intravenous morphine with epidural morphine and observed that use of epidural analgesia is associated with decreased nausea, vomiting, respiratory complications and mechanical ventilation [25,26]. The authors had the same experience in the study cohort. It has also been documented in literature that epidural analgesia is associated with reduced neuro-endocrine surgical stress response after major abdominal surgery [27].
Bosenberg AT et al., reported that the use of epidural in neonates resulted in reduced need for muscle relaxant, opioid analgesics and post-operative ventilator support [28]. Bösenberg AT et al., also reported that neuraxial blockade in neonates is not associated with hypotension and hemodynamic stability is remarkable even in those with congenital heart disease [29]. Recent preclinical studies have demonstrated that use of inhalational agents is associated with increased perinatal neuronal apoptosis and long-term behavioural changes in animal models [30]. The use of epidural analgesia minimises the need for systemic analgesia and prevent such complications.
There is a flip-side to every coin; the use of epidural in neonates in no exception. The authors’ observed that few patients had bradycardia (<100/minute) after giving bolus dose of bupivacaine which was corrected with atropine. There are limited reports in literature describing this procedure and the adversities have been hardly reported (publication bias). Injury to the growing spinal cord is a very genuine concern and cannot be ignored in a zeal for novelty. The relative fluidity of the epidural fat is a definite advantage which allows the anaesthetist to push the catheter to its destination [31]. The use of relatively large-bore catheters for ease of threading and to prevent mal-positioning has also been discussed [32,33]. Multiple techniques have been described to ascertain that the catheter has been positioned correctly such as epidurography [32,34], electrocardiography [35], ultrasonography [36] and trans-oesophageal echocardiography [37]. The use of electrical nerve stimulation through the epidural catheter to delineate the level of tip has also been described [38]. The reports of paraplegia due to intra-spinal hematoma while trying to place a lumbar epidural block may not be ignored [39].
Limitation
The study had its own share of limitations. Firstly, the study-setting being resource-constrained, nitrous oxide and halothane were used as inhalation anaesthetic agents. Second, the possibility of intravenous atropine (administered at induction of anaesthesia) confounding the haemodynamic response of surgery has to be borne in mind. Last but not the least, the position of epidural catheter was not confirmed radiologically.
Conclusion
Use of epidural fentanyl and bupivacaine has been found to be safe and superior to intravenous fentanyl in this study with a potential to offer an awake and comfortable patient at the end of surgery. The observed benefits include obviated requirements for intravenous drugs, early extubation and better pain scores. However, the technique of insertion of epidural catheter neonates is demanding and requires expertise with caution.
aUnpaired t-test, bChi-square test
aChi-square test, bUnpaired t-test, cPaired t-test, *Significant