INTRODUCTION
It is now more than 60 years since the first introduction of the antimalarial drugs Chloroquine (CQ) and Hydroxychloroquine (HCQ) into the management of diseases other than endemic malaria infection. The medication has gained popularity because these agents were observed to be effective against various dermatologic and arthropathic manifestations of rheumatologic disorders, namely, Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjogren’s disease, Dermatomyositis, and so forth. Despite its old history, the drug is still in the market with possible newer indications such as diabetes mellitus, cardiovascular diseases, hematologic malignancies, solid tumours, and antiphospholipid antibody syndrome, as either sole or additive agent [1-5]. The promising applications of these agents make them drugs of future, not past.
Along with increased use of antimalarial agents in rheumatology, a dreadful side effect presented itself; irreversible loss of vision that was first described in the literature by Hobbs and colleagues in 1959 [6]. However, despite concerns regarding the CQ- and HCQ-associated retinopathy, their application in the medical practice has expanded. Hence, appropriate guidelines are needed to address concerns regarding the side effects because the number of patients taking them is growing. This is especially the case for HCQ, which has, superseded CQ in current medical practice.
HCQ is a 4-aminoquinolone antimalarial agent with avidity for pigmented tissues due to binding to melanosomes. Its action is accomplished via unknown mechanisms. Nonetheless, the drug activity is thought to interfere with antigen presentation, cytokine production, and Toll-like receptor signaling via increasing lysosomal pH, which in turn has been linked to reduced proteolytic activity of this intracellular organelle. It seems that the acidic environment of lysosomes is crucial for protonating the HCQ, which is followed by osmotic swelling of lysosomes and increased membrane permeability. The release of enzymes into cytoplasm induces apoptosis which may play an important role in the observed immunomodulatory characteristics of HCQ [7-10]. HCQ has also been categorised as an autophagy inhibitor with new insights in the molecular mechanisms of cancer development, metabolic, and neurodegenerative disorders [11-13].
The goal of this article was to review the effects of HCQ on retina and screening for avoiding its side effects, based on the latest published literature.
Article Search Methodology
In order to prepare this review, authors conducted a search on PubMed for the registered literature. No time filter was applied to our search. Titles were looked containing exact phrases of “hydroxychloroquine” and one of the following words: “toxicity”, “retinopathy”, “mechanism”, and “screening”. The search yielded 95 articles in total, of which, 81 articles were determined relevant to the present work based on their abstracts. Then, authors studied the relevant articles in full and organised the current review according to 58 out of 81 (since the rest of the articles were irrelivent to the topic or were in non english language) which are referenced at the end of this paper.
Review of Literature
Retinal Toxicity
As mentioned above, the most important complication associated with use of HCQ is irreversible retinopathy, though the drug may affect anterior segment structures of the eye in a non-vision-threatening manner. While variable results have been reported in different studies, the HCQ-associated retinal toxicity is estimated to occur in 0.5 to 7.5 percent of patients [14-16]. This wide range is reflective, to some extent, of the difference in the duration of consumption among population studies. Older reports had included patients on short-term treatment while recent reports focused on long-term regimens [17,17]. Nevertheless, the new screening proposals and widespread availability of screening modalities may alter the epidemiological characteristics of HCQ-associated retinal toxicity mostly because more and more patients are screened and diagnosed at an earlier stage.
Presentation
HCQ retinopathy typically presents as retinal atrophy predominantly affecting the macular area. The so-called “bull’s eye maculopathy” is a textbook figure which is neither sensitive nor specific for HCQ retinopathy. It is now only occasionally seen due to increased awareness of the risk and increased screening. Although not fully elucidated, it appears that the damage inflicts mainly the retinal photoreceptor layer with propagation to the Retinal pigment epithelium (RPE). The clinical signs mainly begin in the inferotemporal retina which corresponds to the superonasal visual field [18] and occasional reports of scotomas in very alert patients. On the other hand, recent evidence supports extramacular involvement at least in the Asian population [19,20] which is a challenge to the current screening protocols. We discuss these issues further in the following sections.
Risk Factors
Risk factors associated with the occurrence of CQ/HCQ retinopathy are summarised in [Table/Fig-1] [21]. In general, the role of major risk factors is proven; yet, the participatory role of minor risk factors is not foolproof.
Risk and protective factors for HCQ toxic retinopathy.
| Major risk factors |
|---|
| Overdosing medication above 5 mg/kg (real body weight) per dayAbove 5-years consumptionKidney disease with reduced GFRTamoxifen consumptionMacular disease |
| Minor risk factors |
| Old ageLiver diseaseGenetic predisposition |
| Possible protective factors |
| ABCA4 gene variantsSmokingAlpha-TTP |
HCQ: Hydroxychloroquine; alpha-TTP: Alpha-tocopherol transfer protein
Total Daily Dose
Among major risk factors, total daily dose exerts the greatest effect on the occurrence of retinopathy. It appears that the greater the daily dose, the greater the incidence of retinal damage [22,23]. It is noteworthy that the previous recommendation of maximum prescription doses (6.5 and 3 mg/kg/d for HCQ and CQ, respectively) [15,24] according to ideal body weight, is better replaced by dosing adjusted for real body weight, not only due to ease of calculation, but also due to reduced chance of toxicity observed among thin patients [21,25]. One should not forget that this amount of daily dose only refers to the level below which severe retinal toxicity is less probable. No consensus is present as to the exact safe dose below which the retinal toxicity is precluded [26].
Cumulative Dose
The concept of cumulative dose combines the duration of consumption and the total daily dose parameters into a single predictive factor. If the duration of use is a sole concern, given the safe dose is not violated, the risk is negligible if the drug has been taken for less than 5 years; however, the risk is increased afterwards significantly to 1 percent and reaches to 2 percent after 10 years [25]. According to Melles RB et al., patients taking HCQ above 5 mg/kg/d had 4-fold increased risk of retinopathy after 20 years relative to the risk after 10 years (40 versus 10 percent). In doses between 4-5 mg/kg/d the risk after 20 years of consumption increased 10-fold relative to the risk after 10 years (20 versus 2 percent) [25]. These findings demonstrate that the effects of daily dose should be considered along with the duration of consumption when the probability of retinopathy is being evaluated. Previously, the cumulative dose of 1000 g HCQ and 460 g CQ were considered to correlate with retinopathy. Some authors propose that the cumulative dose concept should be corrected as duration of consumption in relation to daily dose per real body weight.
Renal Function
Renal function should be considered as an important factor while monitoring patients on HCQ. There is a case report of retinal toxicity in renal dysfunction while maintaining even safe-doses [27]. The fact is that about half the metabolism and excretion of HCQ is performed by kidneys, thence, the regular evaluation and proper adjustment of the daily dose should not be undermined [28,29].
Concurrent tamoxifen use: Tamoxifen-associated retinal toxicity has been described first in 1978 by Kaiser-kupfer and Lippman [30]. However, only recently has the synergistic effect of tamoxifen and HCQ retinopathy been reported [31]. The results of a recent study demonstrated a 5-fold increase in the rate of retinal toxicity if both tamoxifen and HCQ were administered simultaneously [25].
Concomitant retinal disease: Another issue that must be contemplated while evaluating HCQ retinopathy is the presence of concomitant retinal diseases. Though the effect of preexisting retinal diseases on HCQ retinopathy has not been evaluated in a well-designed study, it has been proposed that addition of a potentially toxic agent to a vulnerable retina may increase the risk of damage [15]. Additionally, interpretation of screening results is confounded if the retina is previously compromised [25].
Other factors: To a lesser extent, the role of age, liver disease, and genetic polymorphisms has been implicated, though without a clear-cut association [21,24].
Protective Factors
There is no proven protective factor against HCQ retinopathy. However, there are few associations advocated in literature that might provide some protection against toxic effects of HCQ.
Genetic polymorphisms: While earlier investigations of mutations in the ABCA4 gene were suggestive of a predisposing role, a recently published study attributed a protective role for common variants of this gene. Nevertheless, all of these studies are deficient first, by limited number of cases and controls, and second, by only assessing patients taking CQ [32,33].
Smoking: The observation that SLE patients who smoke cigarette are less prone to disease modifying effects of HCQ is interesting [34-36]. It is suggested that smoking interferes with accumulation of HCQ in the lysosomes (a proposed mechanism necessary for antimalarial action) and also induces the metabolic pathway (possibly P450), thus leading to decreased efficacy of HCQ and CQ. Unfortunately, the drug interactions with cigarette smoking may have numerous confounding factors and there is no conclusive data about HCQ-associated retinopathy and smoking [37-39].
Alpha-TTP: Alpha-tocopherol transfer protein (alpha-TTP) is located in all retinal layers. In animal models, it has been demonstrated that the absence of alpha-TTP causes severe CQ retinal toxicity irrespective of vitamin E level status [40].
Screening
In case of HCQ retinopathy, there is no screening method that can detect retinopathy before it is established (stage 1 prevention). One may reckon that appropriate screening would only assist in limiting progressive damage (stage 2 prevention). According to current literature, the sooner the diagnosis is made, the better the outcome in terms of foveal functional loss, if the drug is discontinued in a timely manner [41]. On the other hand, a systematic approach to patients with suspected HCQ-induced retinopathy through properly devised guidelines, not only serves to support withdrawal of the causative agent in case of definite damage, but also prohibits inappropriate discontinuation of the drug in those who need it to control their extra-ocular disease. Furthermore, it may omit unnecessary variations and suboptimal care observed by some investigators [42-44].
In contrast to proponents of universal screening, this approach is criticised by some authorities. Any subject of recommendation for universal screening should encompass the following virtues: first, the cause and effect relationship should be fulfilled; second, there should be (an) available screening method(s) that detect(s) damage at a reversible level or at least at a level that the damage course could be stopped; third, withdrawal of the cause should prevent further damage; fourth, there should be no risk superimposed on the patient by the screening procedures. Addressing the HCQ retinopathy, the first and the last features are agreed. However, it is postulated that by the time screening reveals discernible signs of toxicity, the damage may have gone too far beyond the stage of reversibility. For unknown reasons, the damage at Retinal pigment epithelium (RPE) level may ensue even after the drug has been stopped [45,46]. However, it seems logical that if the diagnosis is made early enough, at least debilitating retinopathy is preventable [41,47]. On the other hand, the cost effectiveness of universal screening is highly debated, when the high prevalence of HCQ use and the relatively low incidence of retinal toxicity are taken into account [44]. It is necessary to consider such issues while selecting groups of patients for screening.
Screening Tools
[Table/Fig-2] summarises all techniques available for screening HCQ retinopathy [21]. Here, we describe the screening methods according to their current status of recommendation.
Screening methods for detection of HCQ retinopathy.
| Subjective | Objective | Recommended | Not recommended | Not clinically widespread |
|---|
| Functional | Structural | Functional | Primary | Secondary | | Microperimetry |
| Automated VF | SD OCT | mfERG | VF | mfERG | Fundus examination | Adaptive optics retinal imaging |
| Microperimetry | FAF | | SD OCT | FAF | Fundus photography | |
| Adaptive optics retinal imaging | | | | TD OCT | |
| | | | | FA | |
| | | | | Full-field ERG | |
| | | | | Amsler grid | |
| | | | | Color vision testing | |
| | | | | EOG | |
VF: Visual field; SD OCT: Spectral-domain OCT; FAF: Fundus autofluorescence; mfERG: Multifocal electroretinogram; TD OCT: Time-domain OCT; FA: Fluorescein angiography; EOG: Electro-oculogram
Comparison of the Recommended Tools
Deciding on which test is the best for screening is difficult and may be unnecessary, given the combination approach that is currently recommended. It appears that sensitivity and specificity of both spectral domain optical coherent tomography (SD OCT) and 10-2 Visual field (VF) perimetry are favourably high enough to be used as screening techniques and, in combination, the resulting sensitivity reaches 89 percent [48,49]. According to Browning DJ et al., the sensitivity of multifocal electroretinography (mfERG) was highest, though least specific, in comparison to other tests, whereas SD OCT acquired the highest specificity with lowest sensitivity [49]. They also reported superiority of adding either SD OCT or 10-2 VF to mfERG instead of combining SD OCT and 10-2 VF. In 2006, Lai TY et al., reported similar sensitivity results for 10-2 VF and mfERG [50]. Kellner U et al., reported a similar efficacy of SD OCT, mfERG, and Fundus autofluorescence (FAF) at detection of early stage HCQ toxicity [51]. In another study, all the recommended screening tools were compared on 10 patients with early, moderate, and severe HCQ retinopathy with quite comparable results in detecting retinal abnormalities, though the mfERG demonstrated better sensitivity at diagnosis of early retinopathy [18]. Cukras C et al., divided 57 patients according to mfERG criteria into two groups of affected and unaffected patients. They provided the population with SD OCT, VF, FAF, and fundus photograph, and reported SD OCT and 10-2 VF to be most specific and most sensitive with reference to the mfERG findings [48].
The following recommendations not only are advocated in the literature, but also take into account the availability and utility in the field of general ophthalmology.
Primary Recommended Techniques
Visual Field Test and OCT
Previously, the white target 10-2 VF testing were described as the single primary screening tool for detection of central and paracentral scotomas in patients on HCQ treatment [52]. Although quite sensitive, the subjective nature and reliability issues inherent with the visual field testing may complicate the interpretation of the results. Subtle changes should not be rejected simply as “insignificant” or “unremarkable” or “nonspecific” and repeat test should be considered in order to detect reproducible changes [24].
Until recently, larger test patterns of 24-2 and 30-2 were regarded ineffective for screening HCQ toxicity due to insufficient central targets [24]. However, in light of current evidence, this recommendation may need revision. Care must be taken not to discard the possibility of HCQ toxicity merely based on 10-2 visual field testing especially among the non-European ancestry. The recent attention to extramacular pattern of toxicity in the Asian population should prompt the ophthalmologist to also look for paracentral scotomas within the 24 or 30 degrees’ visual field, in the non-European descent, until enough epidemiological data regarding ethnical variations in pattern and extent of retinal damage becomes available [19,20].
Although generally less sensitive in comparison to visual field testing, SD OCT is highly specific and its objective time-saving nature makes it an invaluable tool for primary screening [49]. The possible racial variations mentioned above should be considered for the OCT examinations as well, and wider field images could be helpful if clinically relevant. The main feature associated with toxic retinopathy is disruption of the parafoveal photoreceptor layer (the so-called ellipsoid zone) with reduced thickness especially in the inner inferior subfield [18,48]. If possible (considering availability, local policies, and financial concerns) both the visual field and SD-OCT tests should be ordered as primary screening techniques.
Secondary Recommended Techniques
Both mfERG and FAF imaging are useful in confirming the diagnosis of HCQ toxicity. They can be used in conjunction with OCT or VF tests, or as second line modalities if the results of primary screening techniques are borderline.
Multifocal ERG
This is an objective tool that evaluates the localised retinal function with sensitivity profiles at least comparable to that of automated VF [50]. Multifocal ERG results may be used to confirm the equivocal field losses observed in routine VF test and the results are expected to be enhanced if the ring ratio analysis is added [21,31]. Some authorities have reported greatest sensitivity in diagnosing early HCQ-induced retinopathy by mfERG [18,53,54]. It has also been postulated that the mfERG correlates well with the progressive nature of the HCQ-induced toxicity [50]. In a recent systematic review published in 2015, this modality was found to be of greatest sensitivity (90%) and variable specificity [55]. It is efficient at localising subtle changes in the central and paracentral macula, with reduced amplitudes and increased implicit times illustrating the greatest specificity for HCQ retinopathy [47]. Based on a recent study, if the defects are visible on SD OCT, they are already appreciable on mfERG [18]. However, some authors believe that the mfERG is not objective. There is high subjective variation in interpretation with no agreement upon standards for toxicity.
Despite invaluable features that make mfERG gold standard of diagnosis, its limited availability in line with unfamiliarity of primary care ophthalmologists for interpretation of results and cost of the procedure, impose great drawback on its adoption as the primary screening method [43].
Fundus Autofluorescence
This is a gem technique for objectively detecting structural changes in the photoreceptor and RPE layers. Early on, the insult is confined to the photoreceptor layer which appears as a paracentral rim of increased autofluorescence. With progression of the damage and RPE involvement, a prominent change into decreased autofluorescence becomes apparent [56].
Not Recommended Techniques
[Table/Fig-2] lists a number of techniques that are not recommended for screening HCQ toxicity. It is worth mentioning that indirect ophthalmoscopy, as a part of routine ophthalmologic exam, should not be undermined. In fact, basal evaluation of uncomplicated patients starts solely with fundus examination. However, because retinal examination, either by indirect ophthalmoscopy or by serial fundus photographs, remains normal until late in the disease course, and the published data reported nearly one-third normal look retina in patients with established retinopathy, the clinical examination should not be considered as a screening method on the yearly basis [48]. The classic appearance of bull’s eye maculopathy is a manifestation of advanced RPE loss when discontinuing medications may not protect against further injury.
Very recent screening methods, namely, adaptive optics retinal imaging and microperimetry have been proposed but there is still limited data regarding their clinical utility and they are very rarely available. The former can objectively assess the damaged cone structures in early disease. The latter is a very good localising modality of visual field defects, however, similar to automated perimetry, it is hindered by its time consuming and subjective nature.
Discussion
Screening Approach
The screening for HCQ retinopathy may be classified as baseline evaluation and continued evaluation [Table/Fig-3]. A uniform guideline for the screening schedule would reduce the variability in the recommendations presented to patients that may lead to suboptimal care reported by some investigators [42,43]. It also may help better acceptance and liaison by the patient’s part.
The flowchart of screening for HCQ retinopathy.
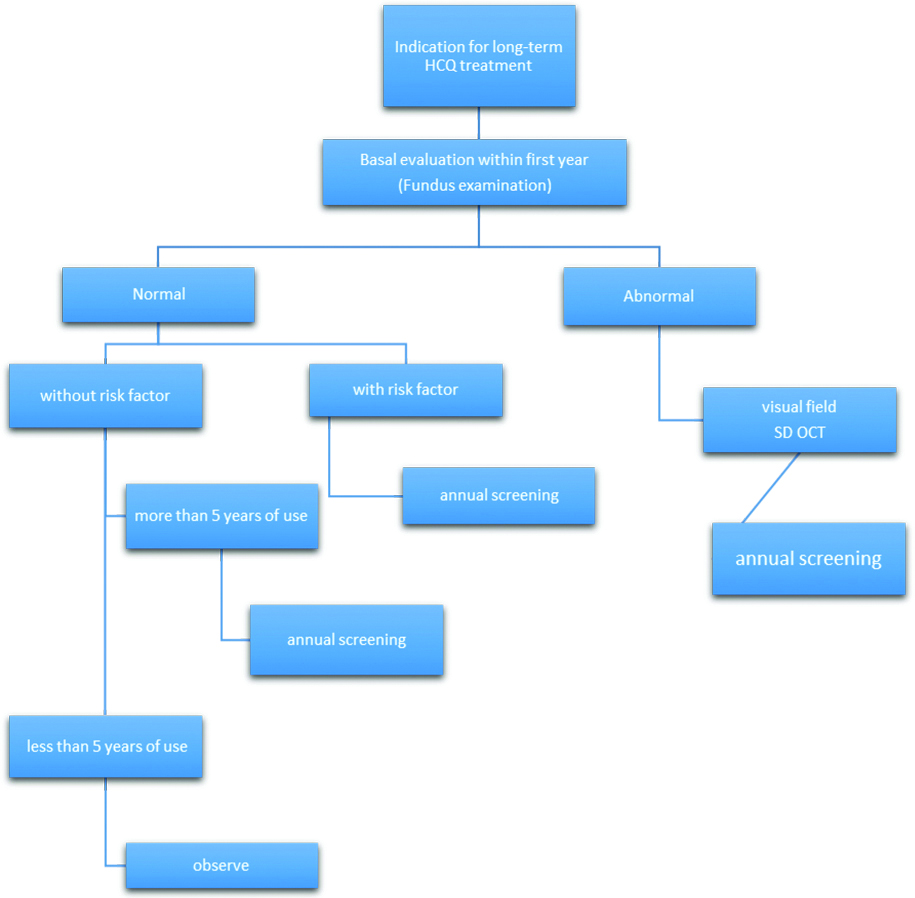
Baseline evaluation: Current recommendation by the American Academy of Ophthalmology (AAO) states that all patient candidates for initiation of a prolonged HCQ treatment plan should visit an ophthalmologist within the first year of treatment, to detect and appropriately document preexisting retinal conditions. If the examination proves normal at the baseline evaluation, the choice for routine VF or OCT testing depends on the preferences of the patient and physician. Yet, any abnormal finding must be investigated, preferentially, by both VF and SD OCT. This approach benefits both patients and physicians to make proper adjustments in the dosing regimen, or, in decision making as to whether the choice of treatment should be altered to other retinal sparing drugs at first instance. It also provides a valuable basis for comparison of the future screening results and may help avoid falsely attributing abnormal screening findings to HCQ-related retinal toxicity.
Continued Evaluation
Due to negligible rate of incidence of HCQ toxicity in the first 5 years of treatment, especially with safe dose regimens, it is recommended to postpone the annual screening until 5 years has passed since the initiation of therapy. This is both cost effective and safe for those who have undertaken basal evaluation and have not been complicated by simultaneous retinal or renal disease. However, the screening threshold should be low if any of the aforementioned major risk factors listed in table 1 are present as they justify annual screening started within 5 years of consumption.
Also, the patient and the prescribing physician should be informed properly so as to any change in the dosing or medical conditions (such as weight loss, kidney disease, liver function abnormality, etc.,) or addition of retinopathic agents such as tamoxifen that may predispose to increased risk of retinal toxicity be reported to the ophthalmologist accordingly if timely steps are to be taken to detect early retinopathy.
Conclusion
Timely screening is invaluable and may be sight saving. If it is possible, the screening plan should be individualised based on the patient’s status and the ophthalmologist’s preferences, considering expertise, local policies, availability of various techniques and their cost, making sure that none of the patient’s interests would be compromised. On the other hand, a uniform guideline for baseline and continued evaluation would reduce the variability in the recommendations and the suboptimal care.
VF: Visual field; SD OCT: Spectral-domain OCT; FAF: Fundus autofluorescence; mfERG: Multifocal electroretinogram; TD OCT: Time-domain OCT; FA: Fluorescein angiography; EOG: Electro-oculogram
[1]. Abdel-Hamid Ahmed AM, El-Firganyl AL, Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokinesJ Mol Hist 2016 47:183-93.10.1007/s10735-016-9664-526872459 [Google Scholar] [CrossRef] [PubMed]
[2]. Sun L, Liu M, Li R, Zhao Q, Liu J, Yang Y, Hydroxychloroquine, a promising choice for coronary artery disease?Medical Hypotheses 2016 93:5-7.10.1016/j.mehy.2016.04.04527372847 [Google Scholar] [CrossRef] [PubMed]
[3]. Koch MW, Zabad R, Giuliani F, Hader W, Lewkonia R, Metz L, Hydroxychloroquine reduces microglial activity and attenuates experimental autoimmune encephalomyelitisJournal of the Neurological Sciences 2015 (358):131-37.10.1016/j.jns.2015.08.152526344560 [Google Scholar] [CrossRef] [PubMed]
[4]. Meroni PL, Prevention & treatment of obstetrical complications in APS: Is hydroxychloroquine the Holy Grail we are looking for?Journal of Autoimmunity 2016 :1-5.10.1016/j.jaut.2016.07.00327496152 [Google Scholar] [CrossRef] [PubMed]
[5]. Lee HO, Mustafa A, Hudes GR, Kruger WD, Hydroxychloroquine destabilizes phospho-s6 in human renal carcinoma cellsPLoS ONE 2015 10(7):e013146410.1371/journal.pone.013146426134285 [Google Scholar] [CrossRef] [PubMed]
[6]. Hobbs He, Sorsby A, Freedman A, Retinopathy following chloroquine therapyLancet 1959 3(2):478-80.10.1016/S0140-6736(59)90604-X [Google Scholar] [CrossRef]
[7]. Wibo M, Poole B, Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1J. Cell Biol 1974 63:430-40.10.1083/jcb.63.2.4304607946 [Google Scholar] [CrossRef] [PubMed]
[8]. Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H, Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytesBiol Pharm Bull 2005 28:947-51.10.1248/bpb.28.94715930724 [Google Scholar] [CrossRef] [PubMed]
[9]. Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquineOncogene 2003 22:3927-36.10.1038/sj.onc.120662212813466 [Google Scholar] [CrossRef] [PubMed]
[10]. Schotte P, Van Criekinge W, Van de Craen M, Van Loo G, Desmedt M, Grooten J, Cathepsin B-mediated activation of the proinflammatory caspase-11Biochem Biophys Res Commun 1998 251:379-87.10.1006/bbrc.1998.94259790964 [Google Scholar] [CrossRef] [PubMed]
[11]. Pasquier B, Autophagy inhibitorsCell Mol Life Sci 2016 73:985-1001.10.1007/s00018-015-2104-y26658914 [Google Scholar] [CrossRef] [PubMed]
[12]. Ruiz A, Rockfield S, Taran N, Haller E, Engelman RW, Flores I, Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosisCell Death and Disease 2016 7:e205910.1038/cddis.2015.36126775710 [Google Scholar] [CrossRef] [PubMed]
[13]. Perez-Neut M, Haar L, Rao V, Santha S, Lansu K, Rana B, Activation of hERG3 channel stimulates autophagy and promotes cellular senescence in melanomaOncotarget 2016 7(16):1991-2004.10.18632/oncotarget.783126942884 [Google Scholar] [CrossRef] [PubMed]
[14]. Wolfe F, Marmor MF, Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosusArthritis Care Res (Hoboken) 2010 62(6):775-84.10.1002/acr.2013320535788 [Google Scholar] [CrossRef] [PubMed]
[15]. Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T, Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practiceArthritis Rheum 1997 40(8):1482-86.10.1002/art.17804008179259429 [Google Scholar] [CrossRef] [PubMed]
[16]. Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisalOphthalmology 2003 110(7):1321-26.10.1016/S0161-6420(03)00409-3 [Google Scholar] [CrossRef]
[17]. Melles RB, Marmor MF, The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapyJAMA Ophthalmol 2014 132(12):1453-60.10.1001/jamaophthalmol.2014.345925275721 [Google Scholar] [CrossRef] [PubMed]
[18]. Marmor MF, Comparison of screening procedures in hydroxychloroquine toxicityArch Ophthalmol 2012 130(4):461-69.10.1001/archophthalmol.2011.37122159170 [Google Scholar] [CrossRef] [PubMed]
[19]. Melles RB, Marmor MF, Pericentral retinopathy and racial differences in hydroxychloroquine toxicityOphthalmology 2015 122:110-16.10.1016/j.ophtha.2014.07.01825182842 [Google Scholar] [CrossRef] [PubMed]
[20]. Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, Pericentral hydroxychloroquine retinopathy in Korean patientsOphthalmology 2015 122:1252-56.10.1016/j.ophtha.2015.01.01425712474 [Google Scholar] [CrossRef] [PubMed]
[21]. Marmor MF, Kellner U, Lai TYY, Melles RB, Mieler WF, Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision)Ophthalmology 2016 123:1386-94.10.1016/j.ophtha.2016.01.05826992838 [Google Scholar] [CrossRef] [PubMed]
[22]. Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF, Rapid onset of retinal toxicity from high-dose hydroxychloroquine given for cancer therapyAm J Ophthalmol 2015 160(4):799-805.e1.10.1016/j.ajo.2015.07.01226189086 [Google Scholar] [CrossRef] [PubMed]
[23]. Navajas EV, Krema H, Hammoudi DS, Lipton JH, Simpson ER, Boyd S, Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host diseaseCan J Ophthalmol 2015 50:442-50.10.1016/j.jcjo.2015.08.00326651304 [Google Scholar] [CrossRef] [PubMed]
[24]. Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF, Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathyOphthalmology 2011 118:415-22.10.1016/j.ophtha.2010.11.01721292109 [Google Scholar] [CrossRef] [PubMed]
[25]. Allahdina AM, Chen KG, Alvarez JA, Wong WT, Chew EY, Cukras CA, Longitudinal changes in eyes with hydroxychloroquine retinal toxicityRetina 2019 Mar 39(3):473-484.10.1097/IAE.000000000000243730741731 [Google Scholar] [CrossRef] [PubMed]
[26]. Brandao LM, Palmowski-Wolfe AM, A possible early sign of hydroxychloroquine macular toxicityDoc Ophthalmol 2016 132(1):75-81.10.1007/s10633-015-9521-y26792426 [Google Scholar] [CrossRef] [PubMed]
[27]. Tailor R, Elaraoud I, Good P, Hope-Ross M, Scott RA, A case of severe hydroxychloroquine-induced retinal toxicity in a patient with recent onset of renal impairment: a review of the literature on the use of hydroxychloroquine in renal impairmentCase Rep Ophthalmol Med 2012 2012:1827-47.10.1155/2012/18274723304587 [Google Scholar] [CrossRef] [PubMed]
[28]. Furst DE, Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseasesLupus 1996 5(1):11-15.10.1177/0961203396005001041 [Google Scholar] [CrossRef]
[29]. Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF, Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseasesInflammopharmacology 2015 23(5):231-69.10.1007/s10787-015-0239-y26246395 [Google Scholar] [CrossRef] [PubMed]
[30]. Kaiser-Kupfer MI, Lippman ME, Tamoxifen retinopathyCancer Treat Rep 1978 62(3):315-20. [Google Scholar]
[31]. Pavlidis NA, Petris C, Briassoulis E, Klouvas G, Psilas C, Rempapis J, Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity: a prospective study of 63 patientsCANCER 1992 69(12):2961-64.10.1002/1097-0142(19920615)69:12<2961::AID-CNCR2820691215<3.0.CO;2-W [Google Scholar] [CrossRef]
[32]. Shroyer NF, Lewis RA, Lupski JR, Analysis of the ABCR (ABCA4) gene in 4-aminoquinoline retinopathy: is retinal toxicity by chloroquine and hydroxychloroquine related to Stargardt disease?Am J Ophthalmol 2001 131(6):761-66.10.1016/S0002-9394(01)00838-8 [Google Scholar] [CrossRef]
[33]. Grassmann F, Bergholz R, Mändl J, Jägle H, Ruether K, Weber BHF, Common synonymous variants in ABCA4 are protective for chloroquine induced maculopathy (toxic maculopathy)BMC Ophthalmology 2015 15:1810.1186/s12886-015-0008-025884411 [Google Scholar] [CrossRef] [PubMed]
[34]. Rahman P, Gladman DD, Urowitz MB, Smoking interferes with efficacy of antimalarial therapy in cutaneous lupusJ Rheumatol 1998 25:1716-19. [Google Scholar]
[35]. Jewell ML, McCauliffe DP, Patients with cutaneous lupus erythematosus who smoke are less responsive to antimalarial treatmentJ Am Acad Dermatol 2000 42(6):983-87.10.1067/mjd.2000.103635 [Google Scholar] [CrossRef]
[36]. Ezra N, Jorizzo J, Hydroxychloroquine and smoking in patients with cutaneous lupus erythematosusClin Exp Dermatol 2012 37:327-34.10.1111/j.1365-2230.2011.04266.x22582908 [Google Scholar] [CrossRef] [PubMed]
[37]. Polet T, The effects of lysosomotrophic amines on protein degradation, migration of nonhistone proteins to the nucleus, and cathepsin D in lymphocytesJ Cell Physiol 1985 122:415-23.10.1002/jcp.10412203122578477 [Google Scholar] [CrossRef] [PubMed]
[38]. Wanwimolruk S, Wong SM, Coville PF, Viriyayudhakorn S, Thitiarchakul S, Cigarette smoking enhances the elimination of quinineBr J Clin Pharmacol 1993 36:610-14.10.1111/j.1365-2125.1993.tb00424.x12959282 [Google Scholar] [CrossRef] [PubMed]
[39]. Schein JR, Cigarette smoking and clinically significant drug interactionsAnn Pharmacother 1995 29:1139-46.10.1177/1060028095029011138573960 [Google Scholar] [CrossRef] [PubMed]
[40]. Shichiri M, Kono N, Shimanaka Y, Tanito M, Rotzoll DE, Yoshida Y, A novel role for ALPHA-Tocopherol Transfer Protein (ALPHA-TTP) in protecting against chloroquine toxicityJ Biol Chem 2012 287(4):2926-34.10.1074/jbc.M111.32128122147702 [Google Scholar] [CrossRef] [PubMed]
[41]. Marmor MF, Hu J, Effect of disease stage on progression of hydroxychloroquine retinopathyJAMA Ophthalmol 2014 132:1105-12.10.1001/jamaophthalmol.2014.109924922444 [Google Scholar] [CrossRef] [PubMed]
[42]. Nika M, Blachley TS, Edwards P, Lee PP, Stein JD, Are long-term chloroquine or hydroxychloroquine users being checked regularly for toxic maculopathy?JAMA Ophthalmol 2014 132(10):1199-208.10.1001/jamaophthalmol.2014.172024970348 [Google Scholar] [CrossRef] [PubMed]
[43]. Au A, Parikh V, Modi YS, Ehlers JP, Schachat AP, Singh RP, Hydroxychloroquine screening practice patterns within a large multispecialty ophthalmic practiceAm J Ophthalmol 2015 160(3):561-68.10.1016/j.ajo.2015.06.00926116260 [Google Scholar] [CrossRef] [PubMed]
[44]. Browning DJ, Impact of the Revised American Academy of Ophthalmology Guidelines regarding hydroxychloroquine screening on actual practiceAm J Ophthalmol 2013 155(3):418-28.10.1016/j.ajo.2012.09.02523218706 [Google Scholar] [CrossRef] [PubMed]
[45]. Farrell DF, Retinal toxicity to antimalarial drugs: chloroquine and hydroxychloroquine: a neurophysiological studyClin Ophthalmol 2012 6:377-78.10.2147/OPTH.S2773122457587 [Google Scholar] [CrossRef] [PubMed]
[46]. Michaelides M, Stover NB, Francis PJ, Weleber RG, Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapyArch Ophthalmol 2011 129(1):30-39.10.1001/archophthalmol.2010.32121220626 [Google Scholar] [CrossRef] [PubMed]
[47]. Maturi RK, Yu M, Weleber RG, Multifocal electroretinographic evaluation of long-term hydroxychloroquine usersArch Ophthalmol 2004 122:973-81.10.1001/archopht.122.7.97315249360 [Google Scholar] [CrossRef] [PubMed]
[48]. Cukras C, Huynh N, Vitale S, Wong WT, Ferris FL, Sieving PA, Subjective and objective screening tests for hydroxychloroquine toxicityOphthalmology 2015 122(2):356-66.10.1016/j.ophtha.2014.07.05625444344 [Google Scholar] [CrossRef] [PubMed]
[49]. Browning DJ, Lee C, Relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathyClin Ophthalmol 2014 8:1389-99.10.2147/OPTH.S6652725114499 [Google Scholar] [CrossRef] [PubMed]
[50]. Lai TY, Ngai JW, Chan WM, Lam DS, Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapyDoc Ophthalmol 2006 112:177-87.10.1007/s10633-006-9006-016804707 [Google Scholar] [CrossRef] [PubMed]
[51]. Kellner S, Weinitz S, Kellner U, Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescenceBr J Ophthalmol 2009 93(11):1444-47.10.1136/bjo.2008.15719819692385 [Google Scholar] [CrossRef] [PubMed]
[52]. Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF, Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy: A Report by the American Academy of OphthalmologyOphthalmology 2002 109(7):1377-82.10.1016/S0161-6420(02)01168-5 [Google Scholar] [CrossRef]
[53]. Lyons JS, Severns ML, Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinographyAm J Ophthalmol 2007 143(5):801-09.10.1016/j.ajo.2006.12.04217336914 [Google Scholar] [CrossRef] [PubMed]
[54]. Penrose PJ, Tzekov RT, Sutter EE, Fu AD, Allen AW Jr, Fung WE, Multifocal electroretinography evaluation for early detection of retinal dysfunction in patients taking hydroxychloroquineRetina 2003 23:503-12.10.1097/00006982-200308000-0001012972762 [Google Scholar] [CrossRef] [PubMed]
[55]. Tsang AC, AhmadiPirshahid S, Virgili G, Gottlieb CC, Hamilton J, Coupland SG, Hydroxychloroquine and chloroquine retinopathy: a systematic review evaluating the multifocal electroretinogram as a screening testOphthalmology 2015 122(6):1239-51.e4.10.1016/j.ophtha.2015.02.01125824328 [Google Scholar] [CrossRef] [PubMed]
[56]. Kellner U, Renner AB, Tillack H, Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquineInvest Ophthalmol Vis Sci 2006 47:3561-68.10.1167/iovs.05-129016877425 [Google Scholar] [CrossRef] [PubMed]