Introduction
A very rare ovarian neoplasm, Struma ovarii, belongs to a class of monodermal and highly specialised teratoma. Histologically, these tumours are composed of benign thyroid tissues with malignant transformation in 0.5 to 5%, according to various studies. Von Kalden described the first case in 1895, but these tumours are still remarkably rare in daily clinical practice and account for close to 3% of ovarian teratomas and 0.3% of all ovarian neoplasms [1]. Although about 15% of the ovarian teratomas partly contain thyroid material, they are not labeled as Struma ovarii unless the amount of thyroid tissue constitutes greater than 50% of the total tumour cells. Because of the rarity of this neoplasm, there is lack of literature concerning its diagnosis and treatment. We aimed to define the clinical features, ultrasonography findings, histological characteristics and optimal management of struma ovarii. On searching our database for all cases of struma ovarii, in a four-year period from January 2014 to December 2017, it was found that 7 patients was with histo-pathological features of struma ovarii. We retrospectively analysed these patients for their signs and symptoms at presentation, results of their investigative work-up, surgery performed and postoperative follow-up.
Case Series
CASE 1
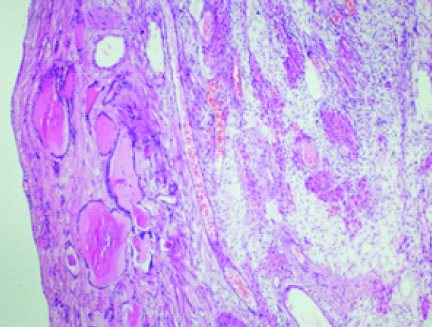
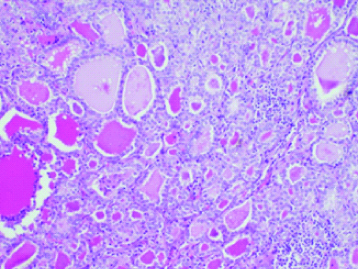
A 62-year-old P4L4 postmenopausal lady presented with right lower abdominal pain since two months and one episode of postmenopausal bleeding. On Ultrasonography, a complex cystic lesion in right adnexa measuring 11x8.5cm was seen with an endometrial polyp. Her CA-125 was 54.9. On Computerised Tomography (CT) scan, a 9×7.8×8.5 cm solid cystic right adnexal mass was noted with a normal left ovary and thickened endometrium with no lymphadenopathy. An endometrial biopsy was done for evaluation of post menopausal bleeding, and it was unremarkable. Staging laparotomy with total abdominal hysterectomy, bilateral salpingo-oophorectomy and infracolic omentectomy was done at our institute suspecting an ovarian carcinoma. She had an uneventful postoperative period and on Histopathological Examination (HPE), ([Table/Fig-1] H&E; 40X and [Table/Fig-2] H&E; 100X), the right ovarian cystic mass was diagnosed as Struma ovarii. All other tissues were found to be free of disease. The patient, on follow-up upto 1 year, required no further intervention.
Histopathology of 62-year-old woman with right ovarian mass showing thyroid tissue along with ovarian follicles (40X).
Histopathology of right ovarian mass in 62-year-old lady showing ovarian stroma with thyroid follicles containing colloid material (100X).
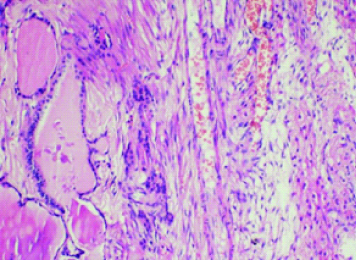
Case 2
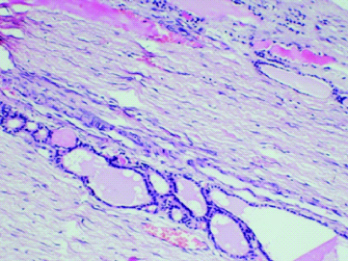
A 36-year-old P2L2 with complaint of abdominal distension of 20 days duration presented to the gynaecological outpatient department of our institute. On Ultrasonography, a multiseptated cyst in pelvis measuring 20×16×11 cm was seen to be arising from right ovary with no evidence of solid nodule and a normal left ovary. Her CA 125 was 14 and CEA-0.68. On CT scan, multiseptated cyst measuring 19.8×17.2 cm with no solid areas similar was noted with mild ascites and a provisional radiological diagnosis of right ovarian cystadenoma was made. The patient was taken up for laparotomy with a plan of frozen section if any suspicious features were found intra-operatively. Right salpingo-oophorectomy was done as the mass had no features suggestive of malignancy intra-operatively. Uterus, left ovary and left tube were found to be normal. Histopathology ([Table/Fig-3], H&E; 100X) revealed Struma ovarii with a normal fallopian tube. The patient, on follow-up upto 1 year, required no further treatment.
A 36-year-old lady with right ovarian mass showing thyroid tissue along with ovarian stroma (100X).
Case 3
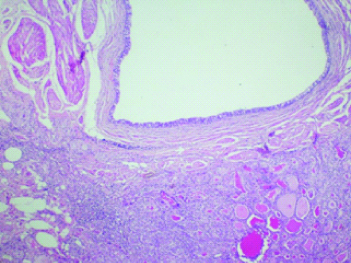
A 28-year-old primiparous lady, being evaluated for secondary infertility at our institute, was incidentally found to have bilateral ovarian cysts suspected to be dermoid cyst on Ultrasonography (Right ovarian cyst measuring 4×4 cm and Left ovarian cyst measuring 4×5 cm). Her CA 125 was 21. Patient underwent a laparoscopic bilateral ovarian cystectomy. On HPE ([Table/Fig-4], H&E; 40X and [Table/Fig-5], H&E; 100X), the cyst wall consisted off sebaceous glands, hair follicles, adipose tissue and thyroid tissue and the diagnosis was struma ovarii of the right ovary. The left ovarian cyst was reported as mature cystic teratoma. On follow-up, upto 1 year, she is doing well.
A 28-year-old lady with right ovarian cystic mass with struma ovarii (40X).
A 28-year-old lady with right ovarian cystic mass showing thyroid follicles containing colloid material (100X).
Case 4
A 55-year-old P3L3 postmenopausal lady presented with complaints of abdominal pain and distension since three weeks. On Ultrasonography, a solid cystic mass arising from left ovary (11×7×5 cm) was seen with a normal right ovary and moderate ascites. Her CA 125 was 234. Staging laparotomy with TAH and BSO with infracolic omentectomy was performed as per the standard management of ovarian tumour. On HPE, malignant struma ovarii with follicular carcinoma was seen in the left ovarian mass and remaining tissues had no disease.
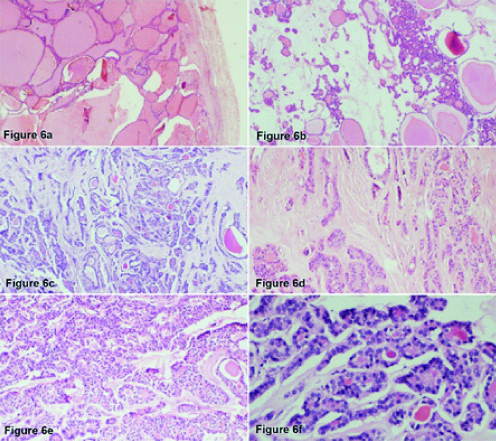
[Table/Fig-6] shows the histopathology images of this case. There are areas showing encapsulated and circumscribed normal thyroid tissue ([Table/Fig-6a] H&E; 20X) along with poorly circumscribed areas with many microfollicles, cords and trabeculae infiltrating into adjacent ovarian parenchyma, amidst few macrofollicles ([Table/Fig-6b,c], H&E; 40X and [Table/Fig-6d], H&E; 100X). At places infiltrating nest and thick trabeculae of tumour cells are seen ([Table/Fig-6e], H&E; 100X). On high magnification, the nuclei show fine chromatin without nuclear overcrowding or pseudoinclusion or nuclear grooving ([Table/Fig-6f], H&E; 200X).
A 55-year-old lady with malignant struma ovarii- histopthology findings (a-20X, b-40X, c-40X, d-100X, e-100X, f-200X).
On immunohistochemistry tumour cells were positive for Thyroid Transcription Factor-1 (TTF-1) and Thyroglobulin and negative for inhibin, calretinin, synaptophysin and chromogranin.
Patient then underwent near-total thyroidectomy at our institute and is presently on levothyroxine 112 mcg and follow-up with Iodine-131 scan. Her last scan done one-month back was negative. Her last Thyroid Stimulating Hormone (TSH) was 0.014 with Thyroglobulin of 0.314. Patient is presently doing well with regular follow-up in radiotherapy department for last 2 years.
Case 5
A 25-year-old unmarried female presented to OPD with complaint of left lower abdominal pain of one-month duration. On Ultrasonography, a 4×3 cm left ovarian cyst, suspected to be dermoid was noted and patient was taken up for laparoscopic left ovarian cystectomy. Histopathology showed lymphocytic thyroiditis and the final diagnosis of struma ovarii was given. Patient required no further intervention.
Case 6
A 46-year-old peri-menopausal P2L2 with complaint of abdominal distension of 2 months duration and marked ascites with right ovarian cyst measuring 5×3 cm and bulky left ovary measuring 4×3 cm on ultrasound underwent TAH with BSO at our institute. HPE showed struma ovarii of the left ovary and mature cystic teratoma of the right ovary. She is presently on follow-up and doing well.
Case 7
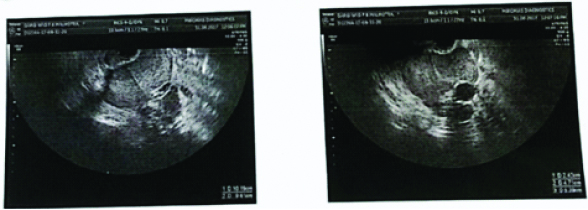
A 63-year-old postmenopausal P1L1, a known diabetic and hypertensive lady had an incidentally detected right adnexal multiloculated cystic mass on USG as shown in [Table/Fig-7], measuring 10.6×9.8 cm with multiple loculi of varying sizes full of internal echoes. Bright foci were seen along the cyst wall (calcification) and ill-defined soft tissue component 14×13 mm in central part (mural nodule). On Magnetic Resonance Imaging (MRI), a large multi-loculated complex cystic mass measuring 9.5×9 cm in pelvis, likely ovarian cystadeno-carcinoma was reported. Her CA125 was 9 and CEA was 0.15. Staging laparotomy with TAH, BSO and infracolic omentectomy was performed as per guidelines and HPE revealed struma ovarii. On regular follow-up upto 1 year, she required no further treatment.
A 63-year-old lady with Ultrasonography showing right adnexal multi-loculated cystic mass.
Discussion
The incidence of struma ovarii differs in various studies. A recent review revealed 2 cases of Struma ovarii (0.7%) among 282 ovarian tumours [2].
According to the previous literature, struma ovarii is mostly benign [3], in our study, 6 out of our 7 cases were benign with only one malignancy. Usually, these tumours are unilateral, all our patients had unilateral tumours. Though the previous literature says that these tumours are more common in right ovary, the incidence was found to be almost equal in right and left in our series. Usually, the size of these neoplasms is said to be lower than 10 cm but can vary from 4 to 25 cm. In our study, the size ranged from 4 to 20 cm.
Age at incidence is usually between ages of 30-50 years, but previous literature has reported a wide range of age distribution between 6-74-year-old [4]-in our series age ranged from 25 to 63 years [Table/Fig-8].
Represents all the cases included in the present series with their age parity, presenting symptoms, surgery performed and histo-pathological findings.
| Case No. | Age | Parity | Presenting symptoms | Surgery performed | HPE findings |
|---|
| 1 | 62 | P4L4 | Abdominal Pain Postmenopausal Bleeding | Staging Laparotomy with TAH+BSO+Omentectomy | Right Ovarian Mass-Struma OVARII |
| 2 | 36 | P2L2 | Abdominal Distension | Laparotomy with Right Salpingo-Oophorectomy | Right Ovarian Cyst – Struma Ovarii |
| 3 | 28 | P1L1 | Incidental | Laparoscopic B/L Ovarian Cystectomy | Right Ovary -Mature Cystic Teratoma With Struma Ovarii |
| 4 | 55 | P3L3 | Abdominal Pain and Distension | Laparotomy with TAH+BSO+OmentectomyNear Total Thyroidectomy | Left Ovary -Malignant Struma Ovarii with Follicular Carcinoma |
| 5 | 25 | Nulliparous (unmarried) | Abdominal Pain | Laparoscopic Left Ovarian Cystectomy | Left Ovarian Cyst -Lymphocytic Thyroiditis, Struma Ovarii |
| 6 | 46 | P2L2 | Abdominal Distension | Laparotomy with TAH+BSO | Left Ovary-Struma Ovarii Right Ovary -Cystic Teratoma (Mature) |
| 7 | 63 | P1L1 | Incidental | Laparotomy with TAH+BSO+ Omentectomy | Right Ovary-Struma Ovarii |
The clinical symptoms with which the patients of struma ovarii present are very diverse according to the previously published case studies. The most common symptom is abdominal pain or pelvic mass. In our series also, abdominal pain and distension were the most common symptoms at presentation [Table/Fig-8].
[Table/Fig-9] Summarises the presenting symptoms of the cases included in the present series. Struma Ovarii can present with clinical features of thyrotoxicosis or thyroid hyperactivity, but majority are inactive [5]. In our study, none of our patients had symptoms of thyroid hyperactivity or deranged thyroid function tests. Serum TSH was performed in all patients as a part of routine pre-operative work-up.
| Lower abdominal pain | 3 |
| Lower abdominal mass | 0 |
| Abdominal distension | 3 |
| Abnormal bleeding per vaginum | 1 |
| Ascites (17% in literature) | 2 |
| Hydrothorax | 0 |
| Asymptomatic (upto 47% in literature) | 2 |
| Thyroid hyperfunction (5-8% in literature) | 0 |
According to the literature, diagnosis before surgery is extremely difficult due to rarity of disease. The Ultrasound, CT, MRI findings are usually nonspecific and show a complex pelvic mass with varying echogenicity. Diagnosis of struma ovarii is suggested by ultasonography in about 11.8% of cases only [6]. MRI routinely shows multiple loculi in cystic mass with varied signal intensity. Few of these loculi show low intensity on T1 weighted images and even lower intensity on T2 weighted images, correlating to gelatinous colloid material pathologically. Elevated CA-125 levels are found infrequently in benign cases of struma ovarii [7], as was the case in the present study. None of the patients had a pre-operative suspicion of struma ovarii though dermoid was suspected in two patients based on ultrasound findings.
The usual differential diagnosis for patients of struma ovarii include Dermoid cyst, benign or malignant cystadenoma which was also the case in our patients. The only preoperative method to diagnose it is the use of scintigraphy Iodine 131 which can show active thyroid tissue in the pelvis [6].
Malignant struma ovarii is found to be extremely rare. One of seven patients in the current series had malignancy containing follicular carcinoma. As per the literature, papillary carcinoma is the commonest malignant struma ovari. Malignant struma ovarii is constituted 70% of the time by papillary thyroid carcinoma. Of this 44% is of classical type and 26% is of follicular variant of papillary thyroid carcinoma. Of late, a new entity of follicular carcinoma called Highly differentiated follicular carcinoma of ovarian origin (HDFCO) has been defined which is discriminated by its extra-ovarian spread of thyroid matter and histological similarity to non-neoplastic thyroid material [8]. Metastasis is unusual (5% to 23% cases).
The factors suggestive of increased risk of malignant behavior are said to be initial extra-ovarian spread, adhesions to surrounding organs, ascites more than 1 liter and strumal size >5 cm.
The final diagnosis is always by HPE. Immunohistochemical markers that aid in the differentiation between benign thyroid tissue and papillary thyroid carcinoma include HBME-1, Cytokeratin 19, and galectin-3. Schmidt J et al., identified multiple molecular abnormalities in thyroid malignancies occurring in ovarian teratomas, including, point mutations in BRAF (V600E and K601E) found in 67% of cases, KRAS, NRAS, Ret/PTC rearrangements and loss of heterozygosity in the PTEN region [9].
Though most authors agree that the treatment for benign cases is surgical removal, but the ideal method of treatment is still under controversy. There is no unanimity for the ideal treatment. Contingent on a literature analysis, the ideal treatment must consider factors including the patient’s age, tumour size, histological malignancy, preservation of fertility, and peritoneal metastasis [10].
The treatment being usually followed is simple unilateral salpingo-oophorectomy for unilateral benign lesions. In bilateral disease and also in women who are postmenopausal, TAH with BSO is recommended. In case of malignant transformation, TAH and BSO with total thyroidectomy and adjuvant ablation is the newer approach being followed [10]. In recurrent or metastatic disease, chemotherapy, suppression of thyroid and external beam radiotherapy are the options available.
In a study by Devaney K et al., 54 patients of struma ovarii were reviewed and classified as “proliferative” struma ovarii (41 cases) and “malignant” struma ovarii (13 cases). In the 13 malignant cases, 11 patients had papillary carcinomas and 2 had follicular carcinomas of thyroid type. Adjuvant therapy was given to none of these patients. The mean interval of follow-up in these patients was 7.3 years and no recurrent disease was seen [11].
DeSimone CP et al., studied 24 patients and 16 of these patients were conservatively followed, whereas 8 patients received additional therapy of varied type (4 patients were treated with I-131). 8 of the conservatively treated patients had recurrence. Thus, they suggest thyroidectomy and treatment with Iodine-131 as the ideal initial treatment for malignant cases of struma ovarii [12].
Yassa L et al., formulated a method for risk stratification of malignant cases of struma ovarii. They stated that thyroid carcinoma measuring lesser than 2 cm, confined to the struma ovarii and having no bothersome histo-pathologic features can be managed as low risk and larger tumours with metastasis, or with aggressive histology can be managed as high risk. In young patients wishing to preserve their fertility, unilateral oophorectomy is adequate if there is no metastasis. Pelvic imaging, thyroxine therapy and serial measurements of serum thyroglobulin are advised when there is a low risk of recurrent carcinoma of thyroid. When there is a high risk of recurrence basing on tumour histopathology, near-total thyroidectomy and radioactive iodine ablation is advised [13].
Janszen EWM et al., for patients with malignant tumour larger than one cm, recommend the ideal option to be total thyroidectomy and 131I ablation in postoperative period. Any detectable serum thyroglobulin after 131I ablation inclines to persistence or recurrence of disease [14].
The prognosis of carcinoma in struma ovarii is hard to determine, owing to its rare occurence and the lack of unanimity in management. Roth LM et al., stated that there was mortality in 100% with undifferentiated (anaplastic) carcinoma, 14% of patients with follicular carcinoma, 7% with papillary carcinoma, and 0% with HDFCO in the literature [15]. But, on the other hand, Robboy SJ et al., after reviewing 88 malignant cases of struma ovarii, realised that even clinically malignant tumours were many times associated with a long survival, as noted by a 25-year survival rate of 84%. They state that no single clinical or histological characteristic reliably predicts which tumours will be biologically malignant unless obviously poorly differentiated, though larger strumal size (>12 cm) and dense fibrous adhesions are indicative of tumours that will have disseminate at the time of surgery [16].
Thus, benign struma ovarii does not require any specific follow-up as per previous studies. In our study follow-up was done till 1 year after surgery. For malignant struma ovarii, risk of recurrence is low, but definite. Possible evaluation for early recurrence includes warning patient about signs of hyperthyroidism, periodic thyroid function tests and Iodine-131 scan. According to the literature review, measurement of thyroglobulin and Iodine-131 whole body scan for 10 years is optimal for follow-up [17]. The sole patient of malignant struma ovarii in our series had a negative I-131 scan and normal thyroglobulin and is presently being followed-up.
Though uncommon, there have been some reports, where, after conservative procedures for malignant cases of struma ovarii, women have had successful pregnancies [18].
Conclusion
Treatment for benign struma ovarii cases is surgery. But the ideal method of management is very controversial. If further pregnancy is desired, conservative management consisting of unilateral oophorectomy or simple cystectomy is deemed to be the ideal treatment. We recommend that conservative treatment must be opted for if malignancy is not suspected and laparoscopic method should be preferred if possible.
The management options for malignancy depend on the disease stage. The first-line surgical options include unilateral oophorectomy; TAH with BSO or just TAH and staging laparotomy. Near-total thyroidectomy and radioactive iodine ablation are the adjuvant treatment options available. Long-term follow-up with thyroglobulin measurement and Iodine-131 scan is advised in all cases.
Larger studies are required to determine an optimal diagnostic method, ideal treatment and protocols for follow-up of this rare ovarian neoplasm.
[1]. Md Nor NB, Kusumoto T, Inoue S, Nakamura K, Seki N, Hongo A, Three cases of struma ovarii underwent laparoscopic surgery with definite preoperative diagnosisActa Med Okayama 2013 67(3):191-95. [Google Scholar]
[2]. Ganga Pilli S, Suneeta KP, Dhaded AV, Yenni VV, Ovarian tumours: A study of 282 casesJ Indian Med Assoc 2002 100(7):420-24. [Google Scholar]
[3]. Mustafa A, Azzam L, Azzam HM, Case report of a struma ovariiAmerican Journal of Medical Case Reports 2016 4(8):272-74. [Google Scholar]
[4]. Dalgaard JB, Wettleland P, Struma ovarii. A follow-up study of 20 casesActa Chir Scand 1956 112:1 [Google Scholar]
[5]. Marcus CC, Marcus SL, Struma ovarii. A report of 7 cases and a review of the subjectAm J Obstet Gynaecol 1961 81:752-62.10.1016/S0002-9378(15)33524-9 [Google Scholar] [CrossRef]
[6]. Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS, Clinical characteristics of struma ovariiJ Gynaecol Oncol 2008 2(19):135-38.10.3802/jgo.2008.19.2.13519471561 [Google Scholar] [CrossRef] [PubMed]
[7]. Loizzi V, Cormio G, Resta L, Fattizzi N, Vicino M, Selvaggi L, Pseudo-Meigs syndrome and elevated CA125 associated with struma ovariiGynaecol Oncol 2005 97:282-84.10.1016/j.ygyno.2004.12.04015790478 [Google Scholar] [CrossRef] [PubMed]
[8]. Roth LM, Karseladze AI, Highly differentiated follicular carcinoma arising from struma ovarii: a report of 3 cases, a review of the literature, and a reassessment of so-called peritoneal strumosisInt J Gynecol Pathol 2007 27(2):213-22. [Google Scholar]
[9]. Schmidt J, Derr V, Heinrich MC, Crum CP, Fletcher JA, Corless CL, BRAF in papillary thyroid carcinoma of ovary (struma ovarii)Am J Surg Pathol 2007 31(9):1337-43.10.1097/PAS.0b013e31802f540417721188 [Google Scholar] [CrossRef] [PubMed]
[10]. Marti JL, Clark VE, Harper H, Chhieng DC, Sosa JA, Roman SA, Optimal surgical management of well differentiated thyroid cancer arising in Struma ovarii: a series of 4 patients and a review of 53 reported casesThyroid 2012 22(4):400-06.10.1089/thy.2011.016222181336 [Google Scholar] [CrossRef] [PubMed]
[11]. Devaney K, Snyder R, Norris HJ, Tavassoli FA, Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 casesInt J Gynecol Pathol 1993 12(4):333-43.10.1097/00004347-199310000-000088253550 [Google Scholar] [CrossRef] [PubMed]
[12]. DeSimone CP, Lele SM, Modesitt SC, Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapyGynecol Oncol 2003 89(3):543-48.10.1016/S0090-8258(03)00141-0 [Google Scholar] [CrossRef]
[13]. Yassa L, Sadow P, Marqusee E, Malignant struma ovariiNature Clinical Practice Endocrinology and Metabolism 2008 4(8):469-72.10.1038/ncpendmet088718560398 [Google Scholar] [CrossRef] [PubMed]
[14]. Janszen EWM, Van Doorn HC, Ewing PC, De Krijger RR, De Wilt JHW, Kam BLR, De Herder WW, Maligne struma ovariiNederlands Tijdschrift voor Geneeskunde 2008 152(29):1647 [Google Scholar]
[15]. Roth LM, Miller III AW, Talerman A, Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literatureInt J Gynecol Pathol 2008 27(4):496-506.10.1097/PGP.0b013e31816a74c618753973 [Google Scholar] [CrossRef] [PubMed]
[16]. Robboy SJ, Shaco-Levy R, Shaco-Levy R, Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spreadInt J Gynecol Pathol 2009 28(5):405-22.10.1097/PGP.0b013e3181a2777719696610 [Google Scholar] [CrossRef] [PubMed]
[17]. Makani S, Kim W, Gaba AR, Struma Ovarii with a focus of papillary thyroid cancer: a case report and review of the literatureGynecol Oncol 2004 94(3):835-39.10.1016/j.ygyno.2004.06.00315350384 [Google Scholar] [CrossRef] [PubMed]
[18]. Ihalagama IR, Hewavisenthi SJ, Wijesinghe PS, Pregnancy following treated malignant struma ovariiCeylon Med J 2004 49(3):90-91.10.4038/cmj.v49i3.324815524228 [Google Scholar] [CrossRef] [PubMed]