The most recent results from Global Cancer Statistics 2018 showed that breast cancer is second most common cancer in both sexes (11.6%), closely after lung cancer, while it is the most commonly diagnosed cancer and leading cause of death from cancer in females worldwide [1]. However, breast cancer is a heterogeneous group of diseases in terms of histology and clinical behaviour, and although there are several molecular subtypes including luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)-type, and triple-negative/basal-like {i.e., Triple-negative breast cancer (TNBC)} tumours [2,3].
Epigenetic alterations of histone are essential in gene transcription pattern regulation in cells, and they are mediated by the catalytic activity of histone deacetylases and methyl- transferees [3-5]. Ezh2 (Enhancer of zeste homolog 2) is a catalytic subunit of PRC2 (Polycomb repressive complex 2), and a histone methyl-transferase. EZH2 facilitates repression of its target genes via tri-methylation of lysine residue 27 on histone 3 (H3K27me3), leading to silencing its target genes involved in cell differentiation and proliferation and cancer progression in multiple malignancies including breast cancer. Mutations and high expression of EZH2 is correlated with tumour grade, metastasis propensity, and poor survival rate in different human cancers [6-8].
Recent studies have shown that EZH2 plays an important role in carcinogenesis and prognosis in the breast, with high expression associated with triple-negative cancer [9]. However, the association of EZH2 expression with Clinicopathological features such as Progesterone Receptor (PR), HER2 expression, nuclear grade and proliferative index are contradictory [10,11].
To date, there have been no reports about EZH2 expression in different immuno-histo-chemical subgroups of breast carcinoma in Iran, specifically considering the associations with EFS and Overall survival (OS). Therefore, the present study aimed to assess EZH2 expression levels in invasive breast carcinoma to determine the association of clinico-pathologic features in different immune-histo-chemical and molecular subgroups with prognosis of breast cancer patients.
Materials and Methods
A cross-sectional analytic survey was performed in Tabriz Haematology and Oncology Research Center, on the specimen of 100 primary breast cancer patients, with follow-up data collected for all of the breast cancer patients from April 2009 to February 2017 (IEC No. 5/d/988072). The pathological characteristics and grading of all breast cancer slides were confirmed by an expert pathologist. The inclusion criteria were any cases with confirmed primary breast cancer in the mentioned study period. They excluded any patient whom death was not due to breast cancer, patients whose pathological samples were unavailable or patients who were not allowed to undergo this research study.
Immunohistochemical (IHC) analysis was performed on paraffin embedded (FFPE) tissue blocks, for biomarkers including EZH2, ER, PR, and HER2. The following primary antibodies were used: EZH2 (Anti-EZH2: AV38470-100UG, QC10904; SIGMA-ALDRICH China), HER/2neu (REF: A0485, 1/200; Dako Denmark A/S), PR (clone PgR636; Dako Denmark A/S), and ER (clone ID5; Dako Denmark A/S, Glostrup, Denmark). The specimens were de-paraffinized through graded alcohols and xylene for IHC analysis. Then, the slides were rinsed in tris-buffered saline (pH=7.6). To block nonspecific binding, Endogenous peroxidase with 3% hydrogen peroxidase was added, and then the samples were incubated with the primary antibodies overnight in 4°C. Then, the slides were incubated in EnVision and with chromogen. Finally, the slides were rinsed in tris-buffered saline, counterstained with haematoxylin, and dehydrated with alcohols (96% and 100%) and xylene before being sealed with a cover slip. 1.15 diluted phosphate-buffered saline solutions for EZH2 antibody was used. Stained cells were scored as the proportion and intensity of EZH2 staining, and the percentage of nuclei that were positively labeled. The total score of proportion and intensity was the final score of expression. Low (range, 0-4) and high (range, 5-8) scores were classified as indicating low and high EZH2 expression, respectively [Table/Fig-1] [12].
Immunohistochemical analysis of EZH2 in breast cancer (×400): (a) Histological image in H&E stain; (b) High; and (c) Low expression.

Molecular Subdivision of Breast Cancer
The four major molecular subtypes of breast cancer defined in most studies, was used as follows: luminal A, luminal B, HER2-type, and triple-negative/basal-like (i.e., TNBC) tumours. Luminal A tumours tend to be ER/PR-positive and HER2-negative. Luminal B tumours are hormone receptor positive (for any of ER, PR) and HER2 positive. High proliferation index was not taken into account in our study. As the names suggests, HER2-type tumours are ER-negative and PR-negative and HER2 positive, whereas TNBCs are negative for ER, PR, and HER2 [2,3].
Clinical Outcome Assessment
The outcomes of interest were breast cancer specific OS and EFS. OS was defined as the time from first diagnosis to the death due to breast cancer or the date of the last follow-up. EFS was measured from the date of first diagnosis to the date of recurrence or metastasis of breast cancer, or the last follow-up date.
Statistical Analysis
Survival function was performed using the Kaplan-Meier method, with log-rank (Mantel Cox) to test the equality of survival distributions for the different luminal subtypes. To assess the effects of variables on OS and EFS, a Cox Regression analysis was then used to give Hazard ratios (HRs) with 95% Confidence intervals (CIs).
The study evaluated the relationship between different molecular and histologic subtypes, different tumour markers, and Clinicopathological aspects with EZH2 expression, as the dependent outcome of interest. Regression analyses were performed in two steps to achieve Odds ratios (ORs) with 95% CIs. IBM SPSS, Version 21 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
Study Participants Characteristics
The paraffin embedded (FFPE) tissue blocks samples were collected for 100 women with primary breast cancers. Most women (n=37, 37%) were aged 51-60 years, with 46% (n=46) aged 50 years or younger and 54 % (n=54) older than 50 years.
By molecular subgroup, 43 cases (43%) had luminal A tumours, 41 cases (41%) had luminal B tumours, 9 cases (9%) had HER2 tumours, and 7 cases (7%) had TNBC tumours. Overall, 74 BCs (74%) had high EZH2 expression, which was most common for the luminal A subtype (n=32, 43.2%) and least common for the TNBC subtype (n=6, 8.1%). There was no significant correlation between subgroups by EZH2 expression (p=0.33).
Association between Molecular Subtypes and EZH2 Expression
Based on simple regression analysis (unadjusted ORs), the TNBC subgroup was twice as likely to have high EZH2 expression compared with the luminal A subgroup (as the reference group) (OR=2.06; 95% CI=0.22 to19.09), and the luminal B subgroup had a 35% reduced likelihood (OR=0.66; 95% CI=0.26 to1.70). There was not any significant relationship between molecular subgroups and EZH2 expression level. Next, we adjusted the data for relevant variables, including the following: age; stage; grade; nervous, lymphatic, and vascular involvement; Oral contraceptive (OCP) intake; breast feeding history; positive family history of breast cancer; and menopausal status. The subsequent multivariate regression analysis showed that different molecular subtypes had no impact on EZH2 expression level. However, lymph node involvement significantly increased the likelihood of high EZH2 expression to 8.6 times compared with cases without nodal involvement (OR=8.62; 95% CI=2.26 to 33.02) (p<0.05). Patients older than 50 years had higher odds of having elevated EZH2 expression compared with patients younger than 50 years (OR=1.29; 95% CI=0.31 to 5.39), and patients with higher disease grades were also more likely to have elevated EZH2 expression (OR=3.33; 95% CI=0.20 to 55.44). The results are summarised in [Table/Fig-2].
Results of uni-variable and multivariable regression analyses of relationship between molecular subtypes and EZH2 expression.
| Variables | EZH2 high expression | Unadjusted regression | | Adjusted regression | |
|---|
| Number | Percent (within group) | OR | 95% CI | p-value | OR | 95% CI | p-value |
|---|
| | | Lower | Upper | | | Lower | Upper | |
|---|
| Age | ≤50 years (n=46) | 37 | 50 | Ref | - | - | - | Ref | - | - | - |
| >50 years (n=54) | 37 | 50 | 0.53 | 0.21 | 1.34 | 0.18 | 1.29 | 0.31 | 5.39 | 0.73 |
| Luminal subtype | A (n=43) | 32 | 43.2 | Ref | - | - | - | Ref | - | - | - |
| B (n=41) | 27 | 36.5 | 0.66 | 0.26 | 1.7 | 0.39 | 0.79 | 0.21 | 2.94 | 0.72 |
| HER2 (n=9) | 9 | 12.2 | - | - | - | - | - | - | - | - |
| TNBC (n=7) | 6 | 8.1 | 2.06 | 0.22 | 19.09 | 0.52 | 1.14 | 0.08 | 17.22 | 0.93 |
| Stage | I (n=13) | 10 | 13.5 | Ref | - | - | | - | - | - | |
| II (n=60) | 43 | 58.1 | 0.76 | 0.19 | 3.1 | 0.70 | 0.12 | 0.02 | 0.96 | 0.04 |
| III (n=12) | 10 | 13.5 | 1.5 | 0.21 | 10.99 | 0.69 | 0.26 | 0.02 | 4.02 | 0.33 |
| IV (n=15) | 11 | 14.9 | 0.83 | 0.15 | 4.63 | 0.83 | - | - | - | - |
| Grade | I (n=12) | 7 | 58.3 | Ref | - | - | | - | - | - | |
| II (n=81) | 61 | 75.3 | 2.18 | 0.62 | 7.63 | 0.22 | 2.86 | 0.53 | 15.42 | 0.22 |
| III (n=7) | 6 | 85.7 | 4.29 | 0.39 | 47.63 | 0.24 | 3.33 | 0.20 | 55.44 | 0.40 |
| Metastasis | Negative (n=83) | 61 | 73.5 | Ref | - | - | - | - | - | - | - |
| Positive (n=17) | 13 | 76.5 | 1.17 | 0.35 | 3.98 | 0.80 | - | - | - | - |
| Lymph node | Negative (n=37) | 18 | 48.6 | Ref | - | - | - | - | - | - | - |
| Positive (n=63) | 56 | 88.9 | 8.44 | 3.06 | 23.33 | 0.00 | 8.62 | 2.26 | 33.02 | 0.00 |
| Lymphatic involvement | Negative (n=66) | 47 | 71.2 | Ref | - | - | | - | - | - | |
| Positive (n=34) | 27 | 79.4 | 1.56 | 0.58 | 4.19 | 0.38 | 1.27 | 0.28 | 5.82 | 0.76 |
| Perineural involvement | Negative (n=61) | 42 | 68.9 | Ref | - | - | | - | - | - | |
| Positive (n=39) | 32 | 82.1 | 2.07 | 0.78 | 5.52 | 0.15 | 1.34 | 0.30 | 6.01 | 0.70 |
| Vascular involvement | Negative (n=21) | 16 | 76.2 | Ref | - | - | | - | - | - | |
| Positive (n=79) | 58 | 73.4 | 0.86 | 0.28 | 2.65 | 0.80 | 0.45 | 0.08 | 2.52 | 0.36 |
| OCP intake | Negative (n=69) | 55 | 79.7 | Ref | - | - | Ref | - | - | - | |
| Positive (n=31) | 19 | 61.3 | 0.40 | 0.16 | 1.02 | 0.05 | 0.24 | 0.06 | 0.99 | 0.05 |
| Breast feeding | Negative (n=14) | 9 | 64.3 | Ref | - | - | | - | - | - | |
| Positive (n=86) | 65 | 75.6 | 1.72 | 0.52 | 5.70 | 0.38 | 1.55 | 0.24 | 10.1 | 0.65 |
| Family history of BC | Negative (n=84) | 64 | 76.2 | Ref | - | - | | - | - | - | |
| Positive (n=16) | 10 | 62.5 | 0.52 | 0.17 | 1.61 | 0.26 | 0.41 | 0.08 | 2.30 | 0.31 |
| Menopausal status | Premenopausal (n=71) | 54 | 76.1 | Ref | - | - | | - | - | - | |
| Postmenopausal (n=29) | 20 | 69.0 | 0.70 | 0.27 | 1.82 | 0.46 | 0.41 | 0.09 | 1.93 | 0.26 |
Lower, lower bound for 95% CI; upper, upper bound for 95% CI; variable adjusted for: age, stage, grade, OCP intake, milking, family history, menopausal status, tumour size, lymph node status, vascular and lymphatic invasion
EZH2: Enhancer of zeste homolog 2; OR: Odds ratio; CI: Confidence interval; OCP: Oral contraceptive; Ref: Reference
Survival Analysis
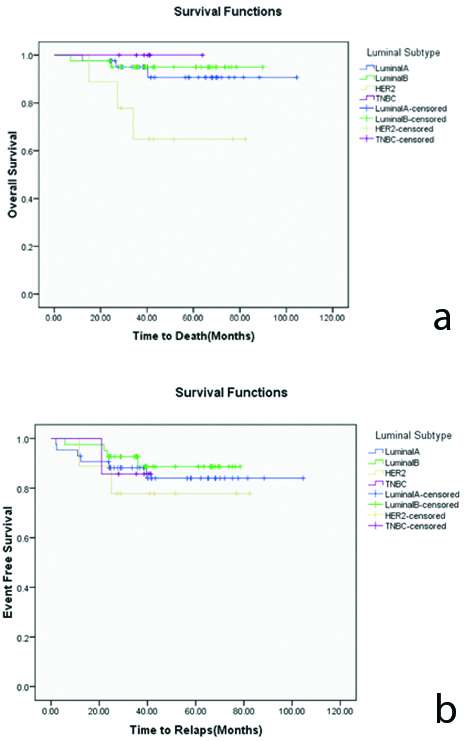
The mean OS was 97.17 months (95% CI=92.23 to 102.11 months) at the end of 5-year follow-up period with an overall mortality rate of 8% (n=8). Survival analysis showed that mortality was highest for the HER2 subtype (n=3, 33.3%), for which the mean OS was 62.52 months (95% CI=44.13 to 80.91 months). In the other groups, the mortality and mean OS rates were, respectively, as follows: 7% (n=3) and 97.59 months (95% CI=89.95 to 105.23 months) for the luminal A subgroup; 4.9% (n=2) and 86.16 months (95% CI=81.14 to 91.19 months) for the luminal B subgroup; and (0%) for TNBC subgroup. The Mantel-Cox log-rank test showed a statistically significant difference in OS by molecular subtype at all-time points (p≤0.05). The survival function plot of OS by molecular subtype is shown in [Table/Fig-2].
Cox’s Regression analysis showed that mortality was about 3 times more in HER2 subtype breast cancers than in the luminal A subgroup (HR=3.16; 95% CI: 1.30-15.45, p<0.005), and 3 times more with peri-neural involvement patients (HR=3.27; 95% CI: 1.55-15.74, p=0.01) [Table/Fig-3].
Results of cox regression analysis of overall survival and disease-free survival in patients with breast cancer.
| Variables | Overall survival | Disease free survival |
|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value |
|---|
| Lower | Upper | | | Lower | Upper | |
|---|
| EZH2 | Low (n=26) | Ref | - | - | - | Ref | - | - | - |
| High (n=74) | 1.35 | 0.12 | 14.90 | 0.81 | 1.55 | 0.25 | 9.61 | 0.64 |
| Age | ≤50 years (n=46) | Ref | - | - | - | Ref | - | - | - |
| >50 years (n=54) | 0.18 | 0.02 | 1.81 | 0.15 | 0.35 | 0.08 | 1.55 | 0.17 |
| Luminal subtype | A (n=43) | Ref | - | - | - | Ref | - | - | - |
| B (n=41) | 0.25 | 0.03 | 2.31 | 0.22 | 0.59 | 0.16 | 2.21 | 0.43 |
| HER2 (n=9) | 3.16 | 1.30 | 15.45 | 0.00 | 1.26 | 0.22 | 7.33 | 0.80 |
| TNBC (n=7) | - | - | - | 0.98 | 0.71 | 0.07 | 6.93 | 0.77 |
| Stage | I (n=13) | Ref | - | - | - | Ref | - | - | - |
| II (n=60) | - | - | - | 0.94 | 1.47 | 0.15 | 14.17 | 0.74 |
| III (n=12) | - | - | - | 0.99 | 5.05 | 1.37 | 8.45 | 0.02 |
| IV (n=15) | - | - | - | 0.94 | 1.78 | 0.15 | 5.74 | 0.45 |
| Grade | I (n=12) | Ref | - | - | - | Ref | - | - | |
| II (n=81) | - | - | - | 0.96 | - | - | - | 0.97 |
| III (n=7) | 1.06 | - | - | 0.99 | - | - | - | 0.99 |
| Lymph node | Negative (n=37) | Ref | - | - | - | Ref | - | - | - |
| Positive (n=63) | 0.14 | 0.01 | 2.09 | 0.15 | 0.76 | 0.15 | 3.77 | 0.74 |
| Lymphatic involvement | Negative (n=66) | Ref | - | - | | Ref | - | - | - |
| Positive (n=34) | 0.98 | 0.06 | 15.13 | 0.52 | 1.58 | 0.33 | 7.58 | 0.57 |
| Perineural involvement | Negative (n=61) | Ref | - | - | - | Ref | - | - | - |
| Positive (n=39) | 3.27 | 1.55 | 15.74 | 0.01 | 0.46 | 0.09 | 2.34 | 0.35 |
| Vascular involvement | Negative (n=21) | Ref | - | - | - | Ref | - | - | - |
| Positive (n=79) | 0.21 | 0.02 | 2.66 | 0.22 | 2.00 | 0.41 | 9.82 | 0.39 |
| Menopausal status | Premenopausal (n=71) | Ref | - | - | - | Ref | - | - | - |
| Postmenopausal (n=29) | 1.66 | 0.05 | 15.66 | 0.78 | 0.85 | 0.12 | 5.84 | 0.87 |
Lower, lower bound for 95% CI; upper, upper bound for 95% CI; variable adjusted for: age, stage, grade, OCP intake, milking, family history, menopausal status, tumour size, lymph node status, vascular and lymphatic invasion
EZH2: Enhancer of zeste homolog 2; HR: Hazard ratio; CI: Confidence interval; OCP: Oral contraceptive; Ref: Reference
Concerning the EFS, 13 (13%) of breast cancer patients had relapsed by the end of the follow-up period. The mean EFS was 92.25 months (95% CI=85.98 to 98.53 months). Relapse was most common with the HER2 subtype (n=2, 22.2% relapse), with a mean EFS of 68.16 months (95% CI=50.64 to 85.69 months). In the other groups, the relapse rates and mean EFS were, respectively, as follows: 14%(n=6) and 90.73 months (95% CI=80.43 to 101.03 months) for the luminal A subgroup; 9.8% (n=4) and 72.29 months (95% CI=66.47 to 78.10 months) for the luminal B subgroup; and 14.3% (n=1) and 38.46 months (95% CI=33.18 to 43.74 months) for the TNBC subgroup. The overall comparison of EFS between groups using the Mantel-Cox log-rank test showed no significant differences (p=0.80). The survival function plot of EFS by molecular subtype is shown in [Table/Fig-2]. The Cox regression models showed that the hazard of relapse was 1.3 times more in the HER2 subgroup than in the luminal A subgroup, but that there were no statistically significance differences in DFS between the different molecular subgroups. Also, after adjusting for all factors, the HRs of relapse were about 2 and 5 times greater for patients with cancer stages III and IV than for those with cancer stage I (HR=1.78; 95% CI: 0.15-5.74, P=0.45) and (HR=5.05; 95% CI=1.37-8.45, p=0.02) respectively [Table/Fig-4].
a) Kaplan-Meier curves showing association of molecular subtypes in patients with breast cancer for overall survival (p≤0.05), b) Kaplan-Meier curves showing association of molecular subtypes in patients with breast cancer for event-free survival (p=0.80).
Discussion
The present study assessed the relationship between EZH2 expression in different molecular subtypes of breast cancer (i.e., luminal A, luminal B, HER2, and TNBC) and patient outcomes. The subsequent multivariate regression analysis showed that different molecular subtypes had no impact on EZH2 expression level. However, based on simple regression analysis, the TNBC subgroup was twice as likely to have high EZH2 expression compared with the luminal A subgroup. Survival analysis showed that mortality was highest for the HER2 subtype (33.3%), and the Mantel-Cox log-rank test showed a statistically significant difference in OS by molecular subtype at all-time points. Also, breast cancer patients with HER2 subtype had significantly worse overall survival, compared with other subgroups. Although relapse was most common with the HER2 subtype (22.2% relapse), the overall comparison of EFS between groups showed no significant differences.
To date, few studies have assessed EZH2 expression by histologic subtype, and to the best of our knowledge, this was the first study in the northwest of Iran. However, the limitation of data sources and time period was lead to small sample size. A pressing clinical problem in breast cancer is the novel and effective treatment to prevent disease progression [13]. To aid in the assessment of clinical progression and prognosis, biomarkers have recently been established. In addition to ER, PR, and HER2 biomarkers, EZH2 over-expression has been reported to be a poor prognostic factor in a few cancers, but particularly in patients with invasive breast carcinoma [14,15].
Researchers have also been studying how the molecular subtypes of breast cancer could aid in the development of new therapies or in treatment planning. The complex profile of each subtype is determined using molecular and genetic information from tumour cells. A few studies have shown that different DNA methylation profiles occur in different subtypes [3,5,16-18], but there has been very limited evidence about the association between EZH2 over-expression and the molecular subtype. In studies of all breast cancer subtypes by Holm K et al., in 2010 and 2012, EZH2 expression was shown to be significantly higher than H3K27me3 expression in HER2 and TNBC subtypes, with significant differences in expression across all subtypes [3,5]. However, the lowest EZH2 expression levels were observed in the luminal A group. Survival analysis also showed poor survival associated with high EZH2 expression in the HER2 and TNBC groups [5]. Similarly, we found a significant difference in OS between subtypes, with the poorest survival outcome seen with the HER2 subtype.
The role of EZH2 over-expression in breast cancer invasion and progression may be caused by EZH2-mediated epigenetic repression of tumour cells. Unsurprisingly, this effect has been shown to work differently depending on the molecular subtype of breast cancer [14]. Guo S et al., demonstrated that TNBC showed the highest EZH2 over-expression, and that EZH2 over-expression was associated with the aggressive pathologic features including high nuclear grade, high proliferative index, and HER2 positivity [9]. In other research, Holm K et al., tested the levels of EZH2 and H3K27me3 in more than 400 tumours by IHC and found significantly high expression of EZH2 in TNBC and HER2-enriched tumours, and significantly high H3K27me3 expression in luminal A, HER2-enriched, and normal-like tumours [5]. Therefore, an abundance of EZH2 was associated with poor disease -free survival.
The ER status is currently thought to be the most important factor in breast cancer, interacting with two different gene transcriptions of EZH2, giving transcriptional activator and repressor roles [19]. This also happens in prostate cancer, which is another endocrine-related cancer in which the key hormone signaling pathways that control tumour growth and differentiation are similar to the different molecular pathways in breast cancer [19]. To develop more effective target therapy for the genetic and epigenetic mechanisms of tumour genesis in hormone-refractory breast or prostate cancer, it is essential that we develop molecular insights into the role of EZH2 over-expression and how this interacts with other hormone receptor mediators.
In a recent study by Roh SG et al., EZH2 over-expression was evaluated in lobular carcinoma insitu and invasive lobular carcinoma. The association of this over-expression with Clinicopathological aspects and its relationship with clinical outcomes were also evaluated [20]. The authors found that EZH2 over-expression has main consequences in breast cancer: 1) poor prognosis and aggressive behaviour; and 2) progression of normal epithelium to malignancy. Specific prognostic factors, namely lymph node status and tumour grade, were also significantly associated with EZH2 expression in their study [20]. Similarly, it was found that breast cancers with positive lymph nodes and higher grades were more likely to have high EZH2 expression, with ORs of 8.62 and 3.3, respectively. The present study results, however, were only statistically significant for lymph node involvement.
The association of EZH2 expression with different tumour markers has been reported in different studies for breast cancer [13,15,21]. However, the mechanism by which EZH2 integrates with different molecular subtypes of breast cancer and its genetic variations requires further investigation. It has been posited that EZH2 expression may switch to become an activator of c-Myc and cyclinD1 (the Wnt signaling pathway) in luminal subtypes (ER-positive cells), or activate nuclear factor-κB target genes in ER-negative cells (basal-like subtypes) [22-24].
Limitation
This study had some important limitations. The small sample size and the limited follow-up period are notable, but we considered that a cohort of 100 patients and a follow-up period of 5 years were adequate for this first study in Iran. We also used IHC for the molecular assay of EZH2 expression because of both the limited availability and the high costs of other molecular techniques.
Future Recommendation
Given that there are very few studies on this topic, we advocate further research with larger sample sizes and the inclusion of molecular techniques.
Conclusion
The study results provide some interesting insights, confirming the prognostic differences by molecular subtypes, in relation to EZH2 protein expression. However, there remains controversy about the prognostic value of different molecular subtypes.
Author’s Contribution
(1) Zohreh Sanaat and Roya Dolatkhah designed of the study supervised the project, abstraction data, and analysis of data. They both prepared the draft of the paper and finalised it based on the comments from the other authors.
(2) Ashraf Fakhrjou participated in the design of study and provided technical support and performed laboratory tests.
(3) Faris Farassati reviewed the drafts of the manuscript and participated in the interpretation of the results and approved the final version.
Declaration
Ethics approval and consent to participate: The study protocol was approved by Tabriz University’s Ethics Committee (License No. 5/d/988072). All the patients signed a consent form during hospitalisation and referring to our clinic, to allow us review their medical records and tissue sample slides, for any future studies.
Availability of data and material: The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests: The authors report no conflicts of interest.
Funding: The authors would like to thank the Haematology and Oncology Research Center for supporting and funding this research study (grant no 95/12). This manuscript submission has been funded by research grant of Ministry of Health and Medical Education, Deputy of Research and Technology for manuscript submission (Grant number: 700/98, 1394/12/24).