In recent years, there has been a reduction in the prevalence of tooth decay and a significant increase in the prevalence of dental erosion in individuals of all age groups and the frequent and excessive consumption of acidic foods is associated with this increase [1].
Dental erosion is defined as the irreversible loss of hard tissue caused by intrinsic acidic sources (perimolysis, is the process whereby gastric acid from the stomach comes into contact with the teeth) and extrinsic acidic sources (high intake of acidic drinks and foods), non-bacterial origin, not directly associated with traumatic factors or cavities [2]. It forms smooth, concave-like injuries on dental surfaces and can interfere with tooth aesthetics and physiology, causing dentin hypersensitivity [3]. It is also worth noting that the appearance of erosive lesions associated with diet depends not only on the acidity of food and beverages, but also on behavioural factors such as the way the liquid is taken to the mouth, the duration of contact with the teeth, swallowing habits and access to saliva [1].
Fruit and vegetable juices are one of the fastest growing segments in the beverages industry, due to concern with health and fitness [4]. There are few studies that address the behaviour and profile of consumers of juices and fruit nectars in Brazilian market, that is growing up vastly [5]. Revenue in the juices segment in Brazil amounts to US$564m in 2018. The market is expected to grow annually by 9.1% (CAGR 2018-2021) [6]. While, soft drinks cause tooth erosion, the population needs to be informed that some fruit juices might not be safe alternatives [7].
Veras AGC and Rocha MPC states that among the most appreciated fruits in the northern and north-eastern regions of Brazil, mainly in the form of juice, the most notable are cupuaçu (Theobroma grandiflorum Willd. ex Spreng. Schum.), soursop (Annona muricate L.), yellow mombin (Spondiasmombin L.) and umbu (Spondiastuberosa Arr. Cam.) [8,9]. Thus, studies that evaluate their action on dental enamel are essential to enable oral health education and prevention programs.
Within this dialectic, and considering the scarcity of data on the properties of juices prepared from Brazilian tropical fruits and their relationship with dental erosion, the objective of this study was to analyse the pH, titratable acidity and erosive potential of the juices made of Theobroma grandiflorum, Annona muricata, Spondias mombin and Spondias tuberosa.
Materials and Methods
It was an experimental study conducted at the Nucleus of Identification and Analytical Quantification (NIQUA) of the Pharmacy School and Dentistry School (Federal University of Juiz de Fora). The duration of the study was three months (December 2016 to February 2017), including literature review, laboratorial experiments, statistical analysis and comprehension of results. The laboratorial experiments (pH analysis and acid titration, preparation of specimens, erosion-remineralization cycling, surface micro-hardness and roughness of samples) lasted five days.
The juices of cupuaçu (Theobroma grandiflorum), soursop (Annona muricata), yellow mombin (Spondias mombin) and umbu (Spondias tuberosa) were prepared using 200 g of fruit pulp purchased in September 2016 at a local supermarket in the city of Belém-Pará, Brazil for 400 mL of ultrapure water (Direct-Q®, Millipore, France) and subsequent homogenization in a domestic blender (HR 2067, Philips Walita, Amsterdam, Netherlands). The juices were kept at room temperature (23°C), stored in glass amber containers, protecting from light and they were used on the same day of preparation.
pH Analysis and Acid Titration
In order to obtain the acidity degree, pH values were checked from 50 mL of each freshly prepared fruit juice at room temperature. The equipment was calibrated using standard solutions of known pH 7.0 and 4.0 (Industrial and electronic commerce Gehaka Ltda, São Paulo, Brazil) and then, the measurement of the juice pH values were made at 25°C using an electronic pH meter connected to a glass electrode (B474, Micronal, São Paulo, Brazil). The samples were estimated in duplicate and showed pH values lower than 7.0, immediately after reading the hydrogen potential, were subjected to acid titration with 1 Mol/L sodium hydroxide (NaOH). The solution was slowly added by using a graduated pipette to raise the pH to neutrality (pH nearly above 7.0) and to analyse the volume necessary to neutralise the acidity of these juices. Magnetic stirrer (Ceramag Midi, Ika, USA), was used under constant stirring, and the pH check-ups were accomplished using an electronic pH meter. In order to determine total titratable acidity, 50 mL of each juice was added by 0.5 mL increments of 1 Mol/L NaOH. The amount of NaOH required to reach pH level of 7.0 was recorded. The titrations for each juice were also repeated twice a day in duplicate to obtain a mean value during 4 days (n=16) [10].
Preparation of Specimens
This study was developed in accordance with the Ethics Committee of the Federal University of Juiz de Fora (n° 921.171).
Fifteen intact human third molars were selected for this study. They were extracted for clinical reasons (such as impaction, pericoronitis, periodontal defects in the distal region of the second molar) and the ease of being obtained from the Human Teeth Bank of the School of Dentistry. It was decided to use third molars guidelines [11,12]. The sample size was based on the methodology established by Sener Y et al., and Lussi L et al., [13,14]. The teeth were disinfected using 5% sodium hypochlorite, for 24 hours, at room temperature [10,13]. With the aid of 11-12 and 13-14 Gracey curettes, the residual tissue adhered to the teeth and the prophylaxis were performed using Robinson brushes in low speed micromotor, pumice stone and ultrapure water. Afterwards, the teeth were submitted to horizontal cuts at the cement/enamel junction, separating the crown from the roots, with a metallographic cutter (Isomet® 1000 Precision Saw, Buehler, USA) and a diamond disc (Diamond wafering Blade, Série 15LC, Buehler, USA). Then, the coronal sections were made in the middle-third under refrigeration and with ultrapure water to avoid cracks, obtaining blocks of enamel with the dimensions of 4×4×2 mm [12] of the flatter region of each tooth [7]. These were fixed in an acrylic resin cylinder so that the vestibular surface was projected outwards [7,12] [Table/Fig-1]. The buccal surfaces were sanded (Politriz PL02, Teclago, Brazil and sandpaper 600, 800, 1200 and 2400) under digital pressure according to Braga, Oliveira and Sobral (2017) with modifications [Table/Fig-2] [11]. Polishing of these surfaces was done with felt disks and diamond pastes (Diamond AC I e II, FGM, Joinville, Brazil). The enamel blocks were washed with ultrapure water in ultrasonic (USC1400, Unique Indústria e Comércio de Produtos Eletrúnicos Ltda, Brazil) bath for 5 minutes; Stored in plastic containers containing absorbent paper moistened with ultrapure water and stored at 4°C until use [7].
Blocks of enamel, with the dimensions of 4×4×2 mm of the flatter region of each tooth, fixed in an acrylic resin cylinder so that the vestibular surface was projected outwards.

The buccal surfaces were sanded under digital pressure.

Erosion-Remineralization Cycling
The study was conducted in an erosion-remineralization cycling model, in order to simulate the conditions in the human oral cavity. The specimens were immersed in artificial saliva, manipulated and obtained from a manipulation Pharmacy (Cavalieri & Ramalho Ltda) {(0.12% KCl; 0.089% NaCl; 0.005% MgCl2; 0.146% Ca3(C6H5O7)2; preservatives (0.002% nipagin and 0.013% nipasol); 1.0% carboxymethylcellulose (CMC); 3.0% sorbitol; distilled water (quantity sufficient for 100 mL)}, according Silva AF et al., [15], at a volume of 2.5 mL/mm2 of the enamel surface, and were maintained under soft agitation for 30 minutes before the first erosive challenge, and then washed in running deionized water [10].
The enamel blocks (n=3) were immersed in the juices: {A-Theobroma grandiflorum; B-Annona muricata; C-Spondias mombin; D-Spondias tuberosa; and E-positive control: 1% citric acid (Cavalieri & Ramalho Ltda)} for 2 minutes without stirring at room temperature and protected from light. This process was performed 5 times a day at 2-hour intervals between daily erosive challenges, lasted for 5 days [Table/Fig-3]. In the interval between the erosive challenges, the samples remained in artificial saliva. Afterwards, they were washed with ultrapure water and dried-up with absorbent paper.
Laboratory experiment-preparation of specimens and erosion remineralization cycling.
Surface Micro-hardness and Roughness of Samples
Before the beginning of the erosion cycle and the sequence cycles, samples were analysed for roughness with a portable surface roughness tester (Surftest SJ-301, Mitutoyo, Japan). Each group was subjected to surface micro-hardness measurement to obtain a baseline value. The hardness value (0.05 kg/mm2) of each specimen was determined using a micro-hardness test (HMV-2, Shimadzu, Kyoto, Japan). The average of these values was used to calculate the percentage of surface hardness loss, using the following formula:
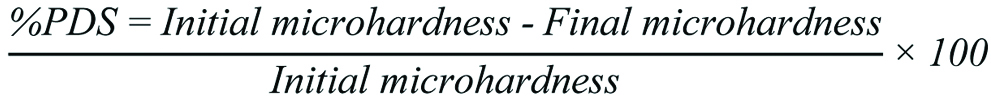
In relation to the roughness of the surface of the samples, the difference between the final roughness and the initial roughness was analysed. Both assays were performed with three specimens and in triplicate.
Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics 22.0 software.wed by post-hoc Tukey’s test. The results were expressed as a mean±the standard error of the mean, and analysed using variance analysis (ANOVA), followed by post hoc tukeys test. The significance accepted was p<0.05.
Results
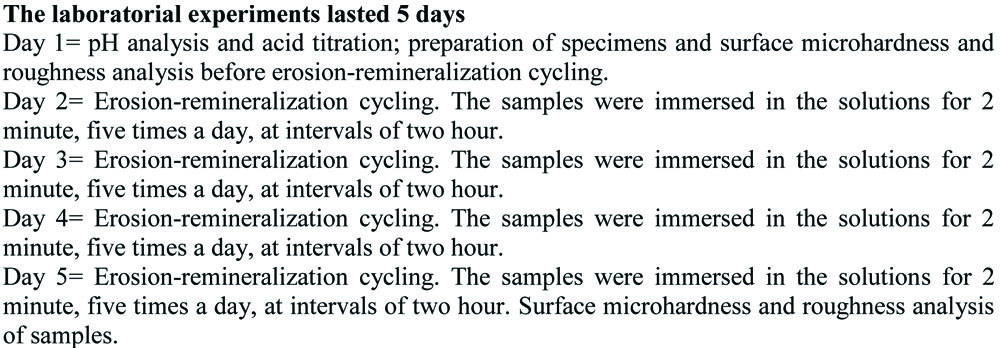
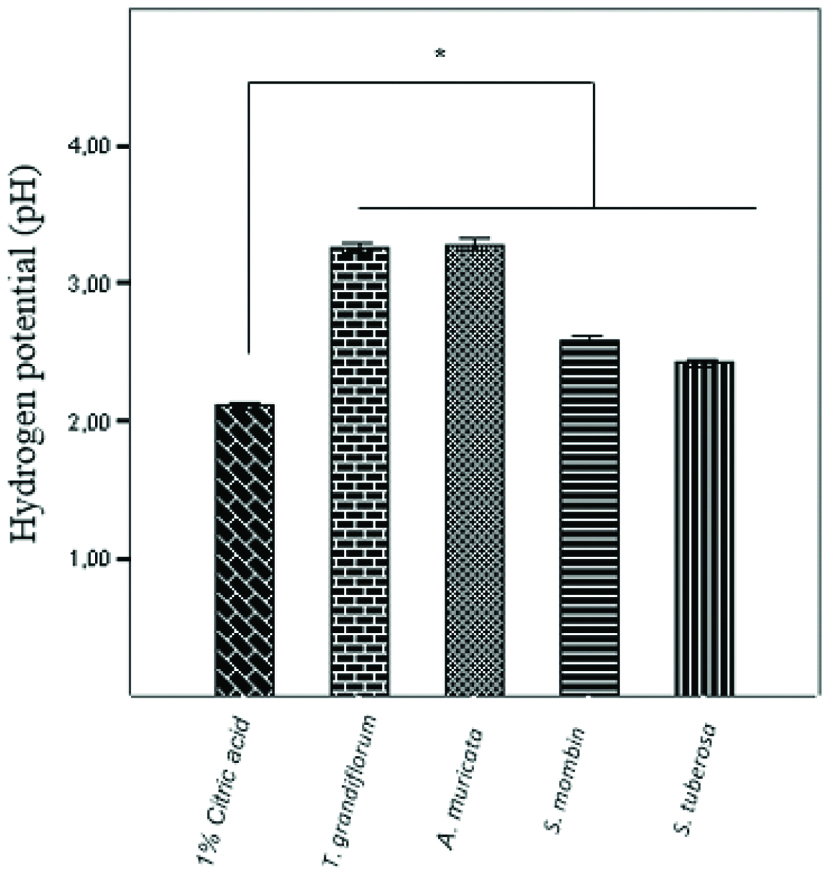
The 1% citric acid (positive control) had a mean pH of 2.10±0.01 and that of the juices varied between 2.41 and 3.28. Although the pH values of the juices were not statistically similar to the control group (p<0.05), they showed lower values than the critical pH for dental enamel demineralization. Still, only the pH of the juices of T. grandiflorum (3.26±0.01) and A. muricata (3.28±0.01) were statistically similar to each other (p=0.881) [Table/Fig-4].
Average hydrogen potential (pH) of the substances tested. Data are presented as mean±SEM of 3 samples/group. *p<0.05 vs. the control group (ANOVA followed by post-hoc Tukey’s test).
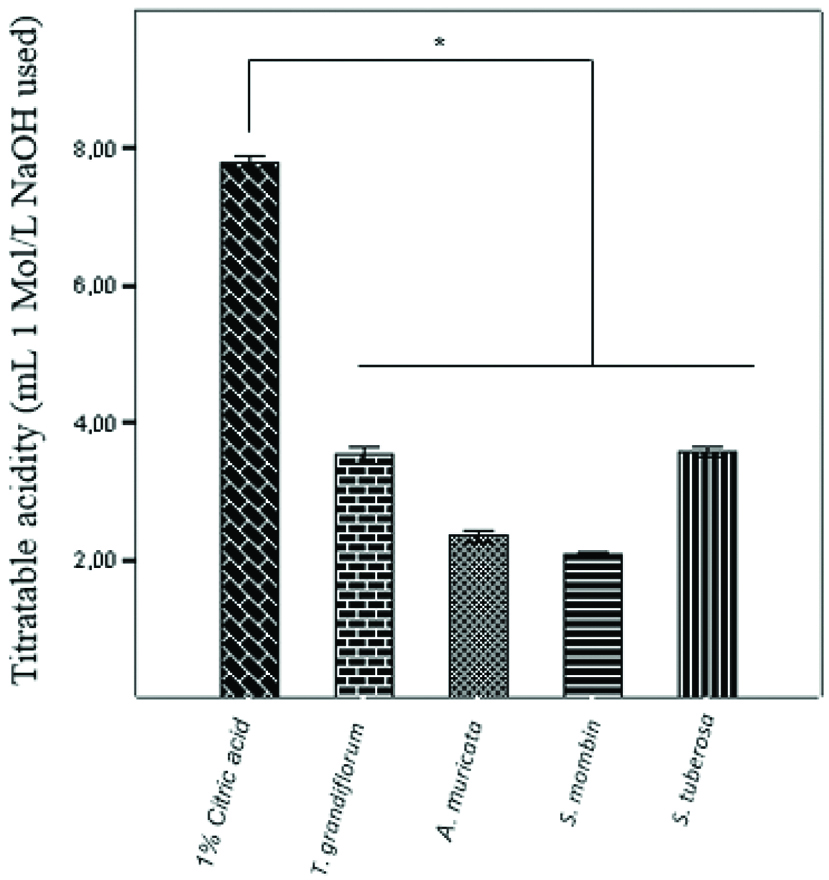
In relation to titratable acidity, the amount of NaOH required to reach neutrality (pH 7.0) followed the following increasing order: S. mombin; A. muricata; T. grandiflorum; and Stuberosa. There was a statistically significant difference between 1% citric acid and the juices analysed. The juices made from T. grandiflorum S. tuberosa were statistically similar to each other (p=0.8555) [Table/Fig-5].
Average titratable acidity of the substances tested. Data are presented as mean±SEM of 3 samples/group. *p<0.05 vs. the control group (ANOVA followed by post-hoc Tukey’s test).
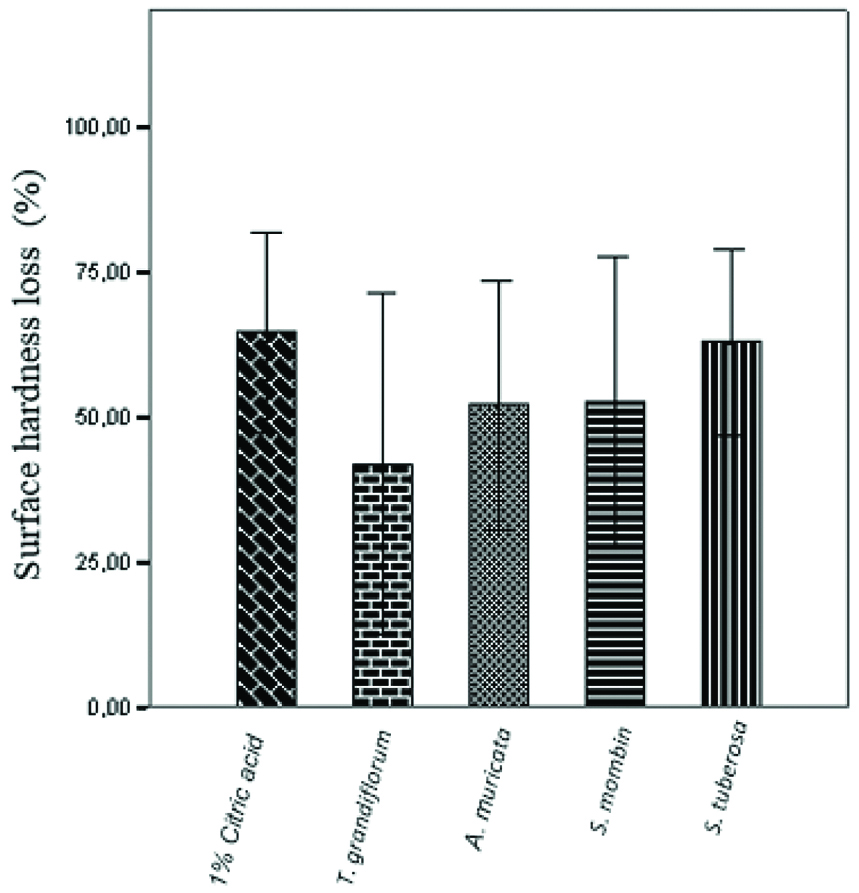
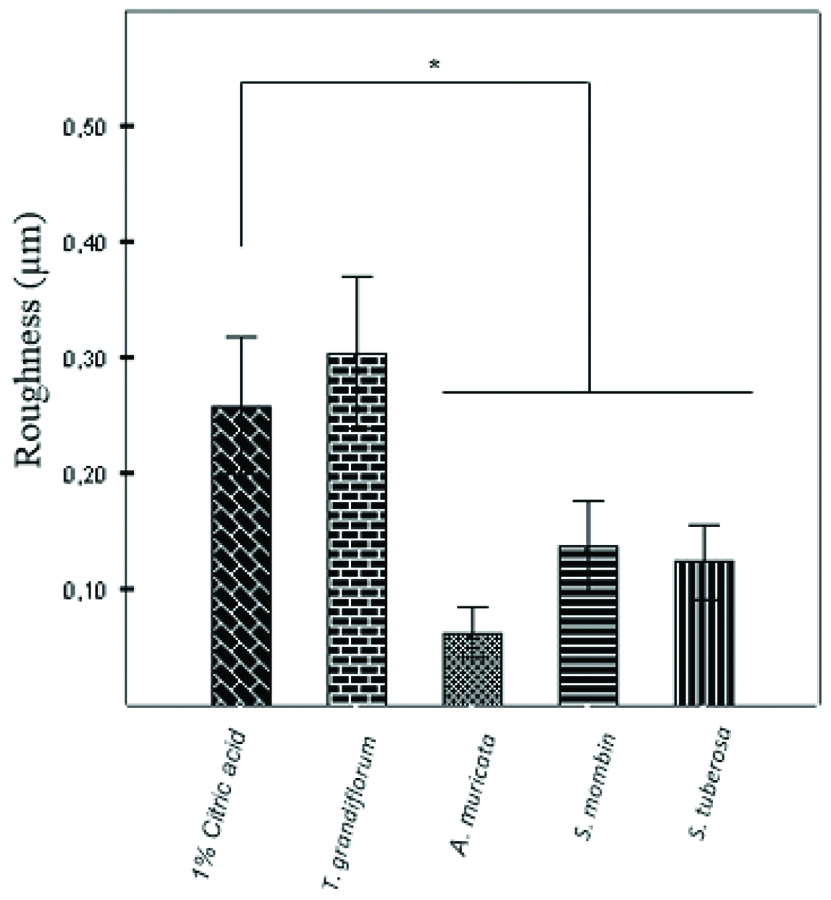
There was no statistically significant difference in the loss of dental surface mineral (micro-hardness) caused by juices (SHL from 41.91 to 62.93%) and 1% citric acid (64.64%) (p>0.05) [Table/Fig-6]. However, in relation to sample surface roughness, there was a statistically significant difference between the control group (0.25 μm±0.02) and the three juices (ranging from 0.06 μm±0.01 to 0.13 μm±0.01, p<0.05), except for the juice of T. grandiflorum (0.30 μm±0.03; p=0.59) [Table/Fig-7].
Difference of the surface micro-hardness of the specimens before and after the erosive challenge. Data are presented as mean±SEM of 3 specimens/group. *p<0.05 vs. the control group (ANOVA followed by post-hoc Tukey’s test).
Difference of the surface roughness of the specimens before and after the erosive challenge. Data are presented as mean±SEM of 3 specimens/group. *p<0.05 vs. the control group (ANOVA followed by post-hoc Tukey’s test).
Discussion
Dental erosion is a result of acid action. Hydroxyl-apatite, one of the components of dental enamel, begins to demineralize naturally when beverage pH is below pH 5.5 [1]. The dynamics of demineralization and remineralization of dental enamel surface depends on the interaction between the erosive challenge characteristics, the biological variability of the environment and the intra-buccal behaviour of the host (such as acidic eating/drinking habits, oral hygiene, reflux, vomiting and medications) [14]. In this study, the pH of all juices analysed was acid, within a narrow range of values from 2.41 to 3.28; similar to the pH of the erosive drinks studied by Barac R et al., [16]. The pH of five different fruit commonly used by people in Northern Brazil, cupuaçu (pH 3.75), soursop (pH 4.02) and yellow mombin (pH 2.75), which this study also evaluates [9]. His findings corroborate those herein, with the yellow mombin having a lower pH than cupuaçu and soursop. Reddy A et al., measured the pH of 380 beverages, all of which presented values lower than the critical pH, 93% of beverages had a pH below 4.0 [17].
The titratable acidity is the quantity of base required to reach neutral pH, a higher titratable acidity is consistent with a higher buffering capacity [18]. As a consequence of the high titratable acidity, there is an increase in the time for the saliva to neutralise the acid, causing differences in the erosive potential of a beverage even within the same pH range. The titratable acidity of the beverages may be influenced by the amount of hydrogen ions in the solution, however, the acid quality, whether weak or strong, with and without chelating, cannot be neglected [8]. For the juices in the present study, titratable acidity values suggest that the beverages tested have pH lower than the critical pH for dental enamel, and have ions capable of interacting with the dental surface and causing tooth tissue loss. It can be assumed that the variation in effect may be due to the different chemical composition of each beverage. Cupuaçu (Theobroma grandiflorum) is a source of flavonoids and, in the Brazilian Amazon, it is used mainly for the production of juice, ice cream, liqueur and jam due to its unique flavour [19]. The soursop (Annona muricata), in turn, has alkaloids, essential oils and acetogenins in its chemical composition [20], which give it several pharmacological properties [20]. Both the yellow mombin (Spondias mombin) and umbu (Spondias tuberosas) contain phenols, tannins, flavones, flavonoids, leucoanthocyanidins and saponins and have healing, antioxidant, antibacterial and anti-inflammatory actions [1].
Out of the methods used to evaluate the demineralization or erosive lesion in the enamel, the surface hardness analysis seems the most adequate one. However, it should be restricted to small changes in the enamel surface [21]. Gonçalves GKM et al., analysed the micro-hardness of dental enamel after exposure to different types of industrialised grape juice [7]. On the fifth day, we analysed all samples and concluded that all of the tested juices led to significant loss of superficial enamel hardness. The effect of four fresh orange juices and fresh fruit juices from two types of oranges on human dental enamel surfaces [22] were evaluated and, as in this study, demonstrated a significant loss of tooth enamel surface hardness caused by acidic beverages. In this study, such loss was statistically similar to that caused by 1% citric acid.
Pereira CAL et al., stated that most of the marketed drinks have high acidity, being able to demineralize the tooth [23]. Such demineralization leaves the teeth more vulnerable to wear and abrasion, increasing the loss of structure of the dental surface, which can be measured by assessing surface roughness. A surface is considered roughened if it is characterised by the presence of high amplitude peaks and valleys with reduced ripple, with 0.2 μm being the surface roughness value considered critical for the retention and adhesion of microorganisms. Regarding the surface roughness of the samples from this study, only 1% citric acid (0.25 μm±0.02) and the juice of T. grandiflorum (0.30 μm±0.03; p=0.59) reached values close to or greater than the critical surface roughness.
In this context, in an attempt to minimise the consequences of the ingestion of fruit juice with erosive potential on the enamel surface, considering its availability to the general public, it has been suggested the use of straws and the addition of some elements to these beverages, such as calcium, phosphate and fluorine, xylitol and fluoride, either alone or in combination [24], as well as green tea [25]. It is also recommended to: drink rationally acid beverages, preferably cold and with the main meals, drink water as the first-choice liquid to quench the thirst, choose to eat fresh fruit as part of a healthy diet; avoid drinking of acidic beverages in baby bottles or during sleep; avoid tooth-brushing soon after ingestion, and use toothpaste with low abrasiveness [1]. These are behaviours of daily routine that help to prevent erosion.
Limitation
The technical difficulty of standardising the sample cuts and the absence of factors that could influence dental erosion, as behaviour of the host-oral hygiene, use of medicaments and others. Despite this, it is suggested that this study provides effective information on the erosive effects of the juices tested, that are widely consumed in Brazil.
Conclusion
The data analysis allows one to conclude that all beverages had the potential of interacting with the dental surface and causing loss of dental tissue. The reduction of tooth enamel micro-hardness and relative increase in surface roughness indicate the erosive potential of Theobroma grandiflorum, Annona muricata, Spondias mombin and Spondias tuberosa fruit juices. In this sense, one should be cautious when drinking such juices, especially in the case of children, who are more susceptible. Also, the dentist should alert patients and in the cases identified in the clinical examination, establish a hygiene and food diet orientation program.