Introduction
Hyperthyroidism is a common endocrine disorder associated with multi-systemic effects, a rarely reported of which is bone loss in newly diagnosed hyperthyroid cases. Elevated thyroid hormones directly stimulate bone cells and accelerate bone turnover. Increased serum Osteocalcin (OSC) levels can be used as a bone biomarker in hyperthyroidism disorder.
Aim
To elucidate the effect of excess thyroid hormone on the serum OSC, alkaline phosphatase, calcium and phosphorus levels in newly diagnosed hyperthyroidism patients.
Materials and Methods
The study was conducted at the National Center of Teaching Laboratories of Medical City Institute, Baghdad, Iraq. Newly diagnosed 50 patients with hyperthyroidism (19 males, 31 females), and 30 age and sex-matched healthy controls (9 males, 21 females) were included in the study. The detailed history of the participants were recorded and thyroid profile including Thyroid Stimulating Hormone (TSH), Total tri-iodothyronine (T3) and Total tetra-iodothyronine (T4) levels, and biochemical markers of bone metabolism-serum OSC, Alkaline Phosphatase (ALP), calcium and phosphorus were analysed. Normality was calculated using Shapiro-Wilk test. Mann-Whitney test was used to determine association among non-parametric data. Independent t-test was used to compare the difference between age groups. Receiver Operating Characteristic curve (ROC curve) was used to identify the validity of markers as an indicator of infection, and the markers were compared according to the area under the curve.
Results
In hyperthyroidism patients, the serum total T4 levels, and T3 levels were significantly elevated (p-value <0.0001), while TSH (p-value <0.0001) were lowered as compared to the control group. Significant difference was observed in serum levels of OSC (p-value <0.0001), ALP (p-value <0.0001) and calcium (p-value=0.0004) levels in hyperthyroidism patients and control group, while no significant (p-value=0.17) difference was observed in serum phosphorus levels. Elevated serum OSC, ALP and calcium were significantly associated with the elevated thyroid hormones (total T3 and total T4).
Conclusion
Association of elevated OSC, ALP and calcium levels with elevated levels of thyroid hormones in hyperthyroid patients indicates that hyperthyroidism influences the bone mineral homeostasis. OSC serves as a better biomarker than the ALP in the risk assessment of bone loss in newly diagnosed hyperthyroidism patients.
Alkaline phosphatase, Calcium, Osteocalcin, Phosphorus, Thyroid hormone
Introduction
Hyperthyroidism refers to excessive synthesis and secretion of thyroid hormone by the thyroid gland. It is characterised by low serum TSH concentrations and increased serum concentrations of thyroid hormones: thyroxine (T4), tri-iodothyronine (T3), or both [1,2]. The thyroid hormones are essential for proper development and differentiation of human body cells, and prevention of bone loss [3]. Normally in adult bone, the processes of resorption and formation are coupled both in space and time, thus bone resorption always precedes formation [4].
Remodeling is essential for bone health, which is coupled with simultaneous formation and resorption of bone. The biochemical markers released during bone remodeling by osteoblasts or osteoclasts can be assessed in blood. Osteocalcin (OSC) is a vitamin-K dependent protein secreted by mature osteoblasts that acts as a bone-derived hormone and depicts bone matrix synthesis [5-7]. Alkaline Phosphatase (ALP) acts as a bone formation marker. Increase levels of serum ALP in hyperthyroidism occur due to functional and structural changes of the bone [8]. Calcium is the product of bone breakdown, and thyroid hormones directly stimulate bone resorption that increases the serum calcium levels along with suppression of parathyroid hormone [9].
In hyperthyroidism, osteoclast and osteoblast activities increases resulting in increased bone turnover. As a consequence, the bone remodeling circle is shortened, but all phases of the cycle are not altered equally. The duration of the formation phase is reduced significantly while the resorption phase remains unaltered. This inability to replace resorbed bone completely, results in 10% net loss of mineralized bone per cycle [3,10]. Thyroid hormones regulate bone development, skeletal growth, and maintenance of the Bone Mineral Density (BMD) [11]. A study on skeletal abnormalities in TSH receptor knockout mice also reported the role of TSH as a direct inhibitor of bone turnover [12].
In hyperthyroid patients, elevated bone formation and bone resorption markers indicates increased bone turnover and osteoclastic bone resorption. This increased mobilisation of bone mineral, often leads to hypercalcemia and hyperphosphatemia [13]. Literature suggests that the thyroid hormone increases the expression of ALP and OSC, and stimulate osteoblast proliferation [14-16]. Based on these findings, the present study was conducted to investigate the influence of hyperthyroidism on biochemical markers of bone metabolism in order to create awareness on risk of development of bone disease in hyperthyroidism.
Materials and Methods
The case-control study was conducted from September 2018 to January 2019, at the National Center of Teaching Laboratories of Medical City Institute, Baghdad, Iraq. Patients attending Outpatient Department of Medical City Hospitals for evaluation of their thyroid status were enrolled in the study. The study was approved by the Ethics Committee of University of Baghdad, Faculty of Pharmacy (IEC registration no. 8A2018). Newly diagnosed 50 (power of study: 70%; confidence interval: 95%) hyperthyroid patients (aged 20-63 years) and 30 age-and sex-matched apparently healthy relatives accompanying the patients were selected. The purpose of the study and nature of all procedures were explained to participants and informed written consent was obtained before commencement of the study.
Patients with co-morbidity (hypo and hyper-parathyroidism, vitamin D deficiency, Cushing’s disease, diabetic nephropathy, inflammatory bowel disease, malabsorptive disease or renal diseases), patients on medication (steroid, bisphosphonates, calcium or vitamin D) influencing bone turnover, or with history of multiple fractures, smoking and caffeine intake were excluded from the study. Patients with a history of thyroid gland surgery or radiotherapy were also excluded.
The diagnosis was based on detailed history and thyroid profile analysis (TSH, total T3, and total T4). Fasting venous blood was collected from patients and controls under the sterile condition for clinical chemistry and hormone analysis. All the serum samples were processed on the same day of collection. The samples were centrifuged at 3500-4000 rpm for 10 minutes to obtain serum. The serum OSC was determined by Chemiluminescent enzyme immunoassay using Immulite 1000 autoanalyser (LKON1Siemens, USA) [17]. The thyroid profile was analysed using Immulite 2000 autoanalyser {TSH (LKRT1), total T3 (L2KT32) and total T4 (LKT41) Siemens, USA} [18-20]. Serum ALP, calcium and phosphorus levels were quantified using DF150 Diemension Rxl Siemens autoanalyser as per International Federation of Clinical Chemistry (IFCC) guidelines, modifications of calcium O-cresolphthalein complex one reaction (OCPC) and classical phosphomolybdate method, respectively [21-23].
Statistical Analysis
Statistical analysis was performed using Statistical Analysis System (SAS; version 9.1). Data were subjected to Shapiro-Wilk test of normality. Mann-Whitney test was used for non-parametric variables. Independent t-test was used to compare the difference between age groups. ROC curve was used to identify the validity of markers as an indicator of infection, and the markers were compared according to the area under the curve. The analysis was performed by using MedCalc Software. The p-value ≤0.05 was considered significant.
Results
Elevated levels of serum thyroid hormones (T3 and T4) and lowered levels of TSH, in addition to the signs and symptoms and physical examination were used for diagnostic criteria for identification of hyperthyroidism patients. Total T3, total T4, total OSC, ALP and Ca+2 were significantly increased in hyperthyroid patients as compared to the control group. However, serum TSH levels in hyperthyroidism patients were significantly (p-value <0.0001) lower than that of control. No significant difference was observed in serum phosphorus levels between patient and control groups (p-value=0.17) [Table/Fig-1].
Thyroid profile and bone biomarker for hyperthyroidism patients and healthy controls.
| Parameters | Control no. 30 Mean±SD | Patients no. 50 Mean±SD | p-value |
|---|
| OSC (ng/mL) | 1.99±0.01 | 8.76±4.25 | <0.0001* |
| TSH (UIU/mL) | 1.94±0.72 | 0.27±0.75 | <0.0001* |
| T3 (nmol/L) | 1.72±0.34 | 3.48±1.34 | <0.0001* |
| T4 (nmol/L) | 128.90±14.39 | 241.42±44.17 | <0.0001* |
| ALP (IU/L) | 93.10±14.38 | 122.76±36.47 | <0.0001* |
| Phosphorus (mg/dL) | 3.72±0.35 | 3.76±0.12 | 0.17 |
| Calcium (Ca) (mg/dL) | 8.68±0.19 | 9.05±0.56 | 0.0004* |
Results are expressed as a Mean±Standard Deviation (SD). Osteocalcin (OSC), Thyroid stimulating hormone (TSH), thyroxine (T4), triiodothyronine (T3), Alkaline phosphatase (ALP). Mann-Whitney test; *p <0.05 Statistically Significant, p<0.001 Statistically highly significant
Data were also subjected to Receiver Operation Characteristic curve (ROC curve) to determine the preferable bone biomarker in the diagnosis of bone disease in hyperthyroidism patients [Table/Fig-2]. Curves of ROC studies confirmed that the T4 was a better diagnostic marker of hyperthyroidism, followed by TSH which are highly sensitive as compare to T3. The sensitivity, specificity and cut-off point of thyroid profile and bone biomarker for hyperthyroidism patients were estimated by ROC [Table/Fig-3]. The cut-off point of T4 was >154 with sensitivity 100, specificity 100 and Area under the curve (AUC) 1.000 [Table/Fig-3,4].
ROC curves for TSH, Total T3, Total T4, OSC, ALP and Ca2+.
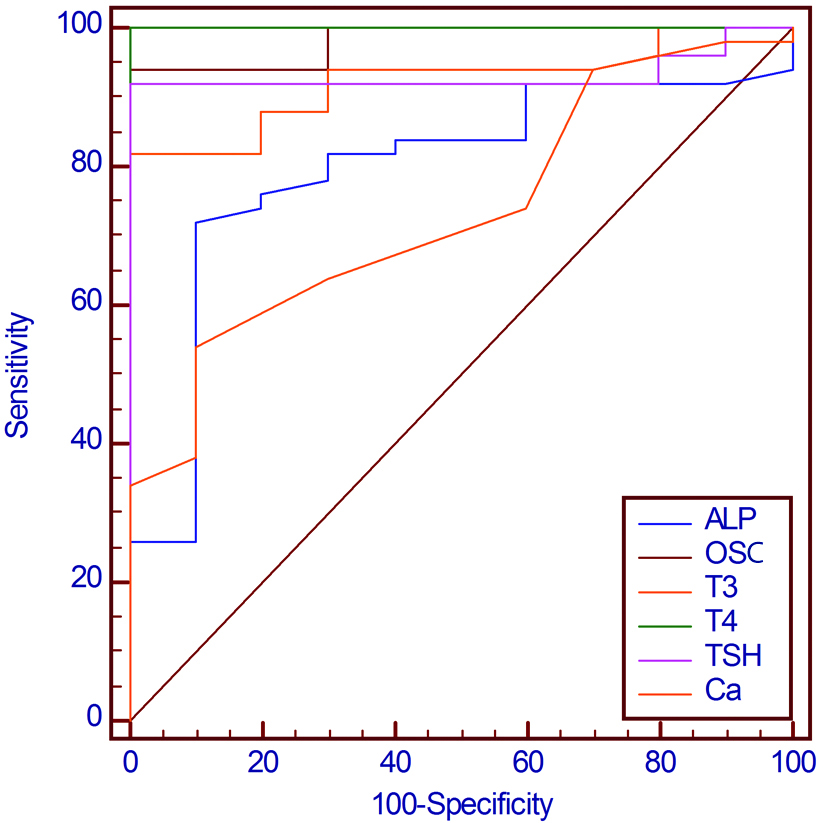
Sensitivity, Specificity and cut-off point of thyroid profile and bone biomarker for hyperthyroidism patients.
| Variables | Sensitivity | Specificity | Cut-off point |
|---|
| TSH | 92.00 | 100.00 | ≤0.67 |
| Total T3 | 82.00 | 100.00 | >2.31 |
| Total T4 | 100 | 100.00 | >154 |
| Osteocalcin | 94.00 | 100.00 | >2.01 |
| Alkaline phosphatase | 72.00 | 90.00 | >104 |
| Calcium | 54.00 | 90.00 | >8.8 |
| Phosphorus | 90.00 | 56.67 | >3.6 |
Area under the curve, Standard error (SE) and 95% confidence intervals (95%CI) for TSH, Total T3, Total T4, OSN, ALP and Ca2+.
| Variable | AUC | SEa | 95% CIb |
|---|
| ALP | 0.795 | 0.0530 | 0.690 to 0.877 |
| OSC | 0.982 | 0.0116 | 0.923 to 0.999 |
| T3 | 0.923 | 0.0303 | 0.841 to 0.971 |
| T4 | 1.000 | 0.000 | 0.955 to 1.000 |
| TSH | 0.932 | 0.0331 | 0.853 to 0.976 |
| Ca | 0.735 | 0.0550 | 0.624 to 0.827 |
Area under the curve (AUC), Standard error (SE), 95% Confidence intervals (95% CI), Osteocalcin (OSC), Thyroid stimulating hormone (TSH), Thyroxine (T4), Triiodothyronine (T3), Alkaline phosphatase (ALP) and Calcium (Ca2+), Mann-Whitney test
aHanley & McNeil, 1982
bBinomial exact
OSC was significantly related to the T4 according to the AUC, indicating that OSC is a better marker for the detection of bone disease in hyperthyroidism patients [Table/Fig-5]. Elevated serum OSC levels were significantly associated with increased total T4 (p-value=0.0208) and total T3 (p-value=0.0457). A significant relationship was also observed between alkaline phosphatase and total T4 (p-value<0.0001), total T3 (p-value=0.0331), TSH (p-value=0.0176) and OSC (p-value=0.0005). The comparison between the calcium and thyroid profile (TSH, T3, and T4) showed a significant relationship between them with p-value=0.0014, p-value=0.0006 and p-value <0.0001, respectively.
Pairwise comparison of ROC curves according to the AUC.
| Comparison between parameters | AUC | p-value |
|---|
| ALP~OSC | 0.187 | 0.0005* |
| ALP~T3 | 0.128 | 0.0331* |
| ALP~T4 | 0.205 | <0.0001* |
| ALP~TSH | 0.137 | 0.0176* |
| ALP~Ca | 0.06 | 0.4100 |
| OSC~T3 | 0.059 | 0.0457* |
| OSC~T4 | 0.018 | 0. 0208* |
| OSC~TSH | 0.050 | 0.142 |
| OSC~Ca | 0.247 | <0.0001* |
| T3~T4 | 0.077 | 0.0110* |
| T3~TSH | 0.009 | 0.8309 |
| T3~Ca | 0.188 | 0.0006* |
| T4~TSH | 0.068 | 0.0399* |
| T4~Ca | 0.265 | <0.0001* |
| TSH~Ca | 0.197 | 0.0014* |
Osteocalcin (OSC), Thyroid stimulating hormone (TSH), Thyroxine (T4), Triiodothyronine (T3), Alkaline phosphatase (ALP) and Calcium (Ca2+). *p<0.05 Statistically Significant, p<0.001 Statistically highly significant.
Discussion
The expedited bone remodeling and reduced bone density in hyperthyroidism increases the risk of fracture and osteoporosis, which can be detected by elevated levels of bone markers [3]. In the current study, the hyperthyroidism patients had highly significant elevated (p-value<0.0001) OSC and ALP levels as compared to that of control group, results of ROC curve confirmed the validation of OSC as a superior marker in the early detection of bone disease in newly diagnosed hyperthyroidism patients. In addition, serum calcium levels were significantly increased, while no significant difference was observed in the serum phosphorus levels. Rai T et al., reported similar findings where increased bone turnover was defined by elevated levels of bone formation markers represented by ALP and OSC and/or serum calcium and phosphorus [24]. Another study revealed that elevated serum levels of OSC and ALP while no significant difference in the serum calcium levels and phosphorus in thyrotoxicosis patients were observed [25]. A similar study by Barsal G et al., reported that bone formation markers (serum ALP and OSC) and bone resorption markers (urinary calcium/creatinine) levels are elevated in hyperthyroidism, which confirms the high turnover state in the skeleton of patients with hyperthyroidism [13]. The accelerated bone remodeling leads to escalate bone resorption, thus increasing release of calcium into systemic circulation. The elevated calcium levels in turn inhibit the Parathyroid Hormone (PTH) secretion and generate a negative calcium imbalance. As a protective response against hypercalcemia, the reduction in PTH levels causes hypercalciuria [26].
In the present study, OSC was found to be correlated with the markers of the osteoblastic process i.e., ALP, a bone formation marker. These findings are in accordance with Zengin Z et al., study [27]. Upon comparison of OSC, ALP and serum calcium with the thyroid profile, a significant relationship between each of these parameters and total T3 and total T4 was observed. However, the exact mechanism of how thyroid hormones affect bone health has not been fully elucidated. Recent studies revealed that T3 increase ALP, OSC and type 1 collagen expression that stimulates and osteoblasts and osteoclasts proliferation [28,29]. Experimental models indicate that T3 act as an anabolic in skeletal development but have catabolic responses in adult skeleton. The knockout mice for thyroid hormone receptor-a isoform showed impairment of growth and reduced BMD [30]. On the contrary, adult knockout mice of thyroid hormone receptor-a showed increased trabecular bone mass and reduced osteoclast number and activity [31].
Triiodothyronine (T3) binds to nuclear receptors and regulates gene transcription via interaction with thyroid hormone response elements of specific genes [32]. T3 amplifies the effects of IL-1 and IL-6, stimulate IL-6 and IL-8, prompts the synthesis of collagen type 1 and OSC. It also regulates bone matrix formation, chondrogenesis bone mineralization, angiogensis and increases proliferation, differentiation, and apoptosis of osteoblasts [33,34]. TSH deficiency in overt hyperthyroidism cases has been reported to be linked to skeletal loss, where TSH act as a direct negative regulator of bone turnover, that hinders osteoblasts differentiation and repress osteoclast formation and survival [35].
Based on the results of the present study and available literature, it can be concluded that elevated serum levels of total T3 and total T4 and low levels of TSH in hyperthyroidism patients cause bone loss, and predisposes the individual to fragility fractures [36,37].
The ROC curve depicts that OSC is a better predictive marker for detection of the osteoblastic activity of bone metabolism in hyperthyroidism. These results were in agreement with other studies reported that elevated levels of OSC correspond to increased osteoblastic activity [38,39].
Hyperthyroidism affects bone health by increasing the rate of bone turnover through the elevation of OSC which is considered a marker of bone turnover. Therefore, early diagnosis and immediate management of hyperthyroidism are essential to prevent bone-related complications.
Limitation
The sample size of the study population is relatively small and post-treatment status of bone mineral metabolism and bone markers were not assessed.
Conclusion
Association of increased serum OSC, ALP and calcium levels with elevated thyroid hormones in hyperthyroid patients indicated that the thyroid hormones influences the bone mineral homeostasis, therefore, the estimation of serum OSC and ALP can help in the early diagnosis of bone diseases in patients with hyperthyroidism. In addition, the present study also suggests that the OSC is the more credible biomarker than the ALP in the bone loss risk assessment in newly diagnosed hyperthyroidism patients.
Results are expressed as a Mean±Standard Deviation (SD). Osteocalcin (OSC), Thyroid stimulating hormone (TSH), thyroxine (T4), triiodothyronine (T3), Alkaline phosphatase (ALP). Mann-Whitney test; *p <0.05 Statistically Significant, p<0.001 Statistically highly significant
Area under the curve (AUC), Standard error (SE), 95% Confidence intervals (95% CI), Osteocalcin (OSC), Thyroid stimulating hormone (TSH), Thyroxine (T4), Triiodothyronine (T3), Alkaline phosphatase (ALP) and Calcium (Ca2+), Mann-Whitney test
aHanley & McNeil, 1982
bBinomial exact
Osteocalcin (OSC), Thyroid stimulating hormone (TSH), Thyroxine (T4), Triiodothyronine (T3), Alkaline phosphatase (ALP) and Calcium (Ca2+). *p<0.05 Statistically Significant, p<0.001 Statistically highly significant.
[1]. De Leo S, Lee SY, Braverman LE, HyperthyroidismLancet 2016 388(10047):906-18.10.1016/S0140-6736(16)00278-6 [Google Scholar] [CrossRef]
[2]. Ali LK, The effect of chronic renal failure on thyroid hormonesIraqi J Pharm Sci 2010 19(1):65-68. [Google Scholar]
[3]. Ale AO, Odusan OO, Afe TO, Adeyemo OL, Ogbera AO, Bone fractures among adult Nigerians with hyperthyroidism: Risk factors, pattern and frequencyJournal of Endocrinology, Metabolism and Diabetes of South Africa 2019 24(1):28-31.10.1080/16089677.2018.1541669 [Google Scholar] [CrossRef]
[4]. Ali SH, Abbass SA, The beneficial role of some bone markers in evaluating women with osteoporosis under different therapeutic regimensIraqi J Pharm Sci 2011 20(1):01-07. [Google Scholar]
[5]. El Hadidy M, Ghonaim M, El Gawad SS, El Atta MA, Impact of severity, duration, and etiology of hyperthyroidism on bone turnover markers and bone mineral density in menBMC Endocrine Disorders 2011 11(1):1510.1186/1472-6823-11-1521819612 [Google Scholar] [CrossRef] [PubMed]
[6]. Greenblatt MB, Tsai JN, Wein MN, Bone turnover markers in the diagnosis and monitoring of metabolic bone diseaseClin Chem 2017 63(2):464-74.10.1373/clinchem.2016.25908527940448 [Google Scholar] [CrossRef] [PubMed]
[7]. Kruger MC, Booth CL, Coad J, Schollum LM, Kuhn-Sherlock B, Shearer MJ, Effect of calcium fortified milk supplementation with or without vitamin K on biochemical markers of bone turnover in premenopausal womenNutrition 2006 22(11-12):1120-28.10.1016/j.nut.2006.08.00817030114 [Google Scholar] [CrossRef] [PubMed]
[8]. Isaia G, Roggia C, Gola D, Di Stefano M, Gallone G, Aimo G, Bone turnover in hyperthyroidism before and after thyrostatic managementJ Endocrinol Invest 2000 23(11):727-31.10.1007/BF0334506111194705 [Google Scholar] [CrossRef] [PubMed]
[9]. Shivaleela MB, Serum calcium and phosphorous levels in thyroid dysfunctionIndian Journal of Fundamental and Applied Life Sciences 2012 2(2):179-83. [Google Scholar]
[10]. Murphy E, Williams GR, The thyroid and the skeletonClin Endocrinol (Oxf) 2004 61(3):285-98.10.1111/j.1365-2265.2004.02053.x15355444 [Google Scholar] [CrossRef] [PubMed]
[11]. Bassett JD, Williams GR, Role of thyroid hormones in skeletal development and bone maintenanceEndocrine Reviews 2016 37(2):135-87.10.1210/er.2015-110626862888 [Google Scholar] [CrossRef] [PubMed]
[12]. Abe E, Marians RC, Yu W, Wu XB, Ando T, Li Y, TSH is a negative regulator of skeletal remodelingCell 2003 115(2):151-62.10.1016/S0092-8674(03)00771-2 [Google Scholar] [CrossRef]
[13]. Barsal G, Taneli F, Atay A, Hekimsoy Z, Erciyas F, Serum osteocalcin levels in hyperthyroidism before and after antithyroid therapyTohoku J Exp Med 2004 203(3):183-88.10.1620/tjem.203.18315240927 [Google Scholar] [CrossRef] [PubMed]
[14]. Sekeroglu MR, Altun ZB, Algun E, Dulger H, Noyan T, Balaharoglu R, Serum cytokines and bone metabolism in patients with thyroid dysfunctionAdv Ther 2006 23(3):475-80.10.1007/BF0285016916912030 [Google Scholar] [CrossRef] [PubMed]
[15]. Cardoso LF, Maciel LM, de Paula FJ, The multiple effects of thyroid disorders on bone and mineral metabolismArq Bras Endocrinol Metabo 2014 58(5):452-63.10.1590/0004-2730000003311 [Google Scholar] [CrossRef]
[16]. Tsevis K, Trakakis E, Pergialiotis V, Alhazidou E, Peppa M, Chrelias C, The influence of thyroid disorders on bone density and biochemical markers of bone metabolismHorm Mol Biol Clin Investig 2018 35(1)10.1515/hmbci-2018-003930218603 [Google Scholar] [CrossRef] [PubMed]
[17]. Fassbender WJ, Steinhauer B, Stracke H, Schumm-Draeger P-M, Usadel K-H, Validation of a new automated immunoassay for measurement of osteocalcinClin Lab 2002 48:31-38. [Google Scholar]
[18]. Bayer M, Kriss JP, McDougall IR, Clinical experience with sensitive thyrotropin measurement: Diagnostic and therapeutic implicationJ Nuclear Med 1985 36:1248-56. [Google Scholar]
[19]. Hollander CS, Shenkman L, Radioimmunoassays for triiodothyronine and thyroxine. In:Rothfeld B, editorNuclear medicine in vitro 1974 PhiladeliphiaLippincott:136-49. [Google Scholar]
[20]. Britton KE, Quinn V, Brown BL, Ekins RP, A strategy for thyroid function testsBr Med J 1975 iii:350-52.10.1136/bmj.3.5979.3501156751 [Google Scholar] [CrossRef] [PubMed]
[21]. Bowers GN, McComb RB, A continuous spectrophotometric method for measuring the activity of serum alkaline phosphataseClin Chem 1966 12:70 [Google Scholar]
[22]. Stern J, Lewis WHP, The colorimetric estimation of calcium in serum with O-cresolphtalein complexoneClin Chim Acta 1957 2:576-80.10.1016/0009-8981(57)90063-3 [Google Scholar] [CrossRef]
[23]. Daly JA, Ertingshausen G, Direct method for determining inorganic of phosphate in serum with the centrifichemClin Chem 1972 18:263-65. [Google Scholar]
[24]. Rai T, DSA J, Rai S, Bone turnover markers; An emerging tool to detect primary osteoporosisJournal of Clinical & Diagnostic Research 2018 12(12):BC04-BC07. [Google Scholar]
[25]. Ale AO, Ogbera AO, Ebili HO, Adeyemo OL, Afe TO, Prevalence, predictive factors, and characteristics of osteoporosis in hyperthyroid patientsInt J Endocrinol 2018 (3):1-7.10.1155/2018/354025629849614 [Google Scholar] [CrossRef] [PubMed]
[26]. Navikala K, Vasudha KC, Kalra P, Radhika K, Can serum osteocalcin level be used as a marker to assess bone remodeling status in hyperthyroidism?Int J Biochem Res Rev 2016 10(3):1-9.10.9734/IJBCRR/2016/23450 [Google Scholar] [CrossRef]
[27]. Zengin Z, Sertkaya AC, The effect of hyperthyroidism on bone mineral density in premenopausal womenJ Thyroid Disord Ther 2012 1(1):10410.4172/2167-7948.1000104 [Google Scholar] [CrossRef]
[28]. Williams GR, Bassett JH, Thyroid diseases and bone healthJ Endocrinol Invest 2018 41(1):99-109.10.1007/s40618-017-0753-428853052 [Google Scholar] [CrossRef] [PubMed]
[29]. Bakos B, Takacs I, Stern PH, Lakatos P, Skeletal effects of thyroid hormonesClin Rev Bone Miner Metab 2018 16(2):57-66.10.1007/s12018-018-9246-z [Google Scholar] [CrossRef]
[30]. Bassett JH, Nordström K, Boyde A, Howell PG, Kelly S, Vennström B, Thyroid status during skeletal development determines adult bone structure and mineralizationMol Endocrinol 2007 21(8):1893-904.10.1210/me.2007-015717488972 [Google Scholar] [CrossRef] [PubMed]
[31]. O’Shea PJ, Harvey CB, Suzuki H, Kaneshige M, Kaneshige K, Cheng SY, A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormoneMolecular Endocrinology 2003 17(7):1410-24.10.1210/me.2002-029612677005 [Google Scholar] [CrossRef] [PubMed]
[32]. Doga M, Formenti AM, Frara S, Memo M, Giustina A, Mazziotti G, Subclinical thyrotoxicosis and boneCurr Opin Endocr Metab Res 2018 3:25-30.10.1016/j.coemr.2018.03.002 [Google Scholar] [CrossRef]
[33]. Basset J, Williams G, The molecular actions of thyroid hormone in boneTrends Endocrinol Metab 2003 14:356-64.10.1016/S1043-2760(03)00144-9 [Google Scholar] [CrossRef]
[34]. Tuchendler D, Bolanowski M, The influence of thyroid dysfunction on bone metabolismThyroid Res 2014 7(1):1210.1186/s13044-014-0012-025648501 [Google Scholar] [CrossRef] [PubMed]
[35]. Agrawal M, Zhu G, Sun L, Zaidi M, Iqbal J, The role of FSH and TSH in bone loss and its clinical relevanceCurr Osteoporos Rep 2010 8(4):205-11.10.1007/s11914-010-0028-x20809202 [Google Scholar] [CrossRef] [PubMed]
[36]. Murphy E, Gluer CC, Reid DM, Felsenberg D, Roux C, Eastell R, Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal womenJ Clin Endocrinol Metab 2010 95(7):3173-81.10.1210/jc.2009-263020410228 [Google Scholar] [CrossRef] [PubMed]
[37]. Ercolano MA, Drnovsek ML, Silva Croome MC, Moos M, Fuentes AM, Viale F, Negative correlation between bone mineral density and TSH receptor antibodies in long-term euthyroid postmenopausal women with treated Graves’ diseaseThyroid Res 2013 6(1):1110.1186/1756-6614-6-1124020400 [Google Scholar] [CrossRef] [PubMed]
[38]. Akalin A, Colak Ö, Alatas Ö, Efe B, Bone remodelling markers and serum cytokines in patients with hyperthyroidismClin Endocrinol (Oxf) 2002 57(1):125-29.10.1046/j.1365-2265.2002.01578.x12100080 [Google Scholar] [CrossRef] [PubMed]
[39]. Kisakol G, Kaya A, Gonen S, Tunc R, Bone and calcium metabolism in subclinical autoimmune hyperthyroidism and hypothyroidismEndocrine Journal 2003 50(6):657-61.10.1507/endocrj.50.65714709834 [Google Scholar] [CrossRef] [PubMed]