COPD is a progressive respiratory disorder characterised by airway obstruction; recurrent symptoms include cough, sputum production, breathlessness and impaired exercise tolerance [1]. The prevalence of depression in COPD subjects varies significantly in various studies, from 8 to 80%; higher when compared with gender and age-matched healthy subjects [2-5]. Compared to other respiratory and infectious diseases like tuberculosis, psychiatric comorbidities are more common in patients with COPD due to its limitation in exercise capacity and chronicity [6]. Anxiety and depression are risk factors for re-hospitalisation in these patients [7,8]. Depression is a substantial risk for increased mortality, although the mechanism needs further research [9].
Studies have found a negative association between depression and the levels of physical activity [10-12]. However, the link is bidirectional since physical activity also improves the symptoms of depression even without any medical intervention [13]. Hence, the role of depression in exercise limitation in COPD subjects is not clearly understood and needs further research; while few studies depicted the relationship between depression and reduced exercise capacity in COPD [14,15] others rejected it [16,17].
Depression and COPD share some common clinical outcomes, such as impaired physical activity and quality of life. The recognition of this dangerous link may be clinically important as a potential target to improve patients’ quality of life. The present study was aimed at evaluating the levels of depression, pulmonary function, symptoms, Length of stay (LOS), quality of life of COPD patients and physical activity in COPD subjects. The secondary outcome of the study was to find out the determinants of impaired physical activity in COPD subjects by multivariate analysis.
Materials and Methods
Study Design
It was a cross-sectional type of analytical study conducted in SHKM GMC Nalhar hospital, from July 2018 to March 2019. Consecutive out patients attending the Chest Outpatient Clinic aged (between 40-80 years) and diagnosis of COPD, who met the inclusion criteria were sequentially recruited for the study. The diagnosis of COPD was established based on complete medical history, symptoms, signs and available pulmonary function tests, as per the standard definitions provided by GOLD (Global Initiative for Chronic Obstructive Lung Disease, 2018) guidelines [18]. Since the FEV1 value decreases more quickly with age than the FVC, the GOLD definition tends to over diagnose COPD in the elderly and underdiagnose in young population. Therefore, sampling population was included from age group of 40-80 years in this study.
The study was conducted adhering to the guidelines of the Declaration of Helsinki as well as approval was sought by the Institutional ethical committee (Letter No.-SHKM/IEC/2018/8). Also, written informed consent was obtained from all patients participating in the study.
Inclusion and Exclusion Criteria
Inclusion criteria were: (1) post bronchodilator (400 microgram Salbutamol) ratio of Forced Expiratory Volume in one second to Forced Vital Capacity <0.70 (FEV1/FVC<0.70); (2) stable conditions i.e., absence of exacerbation (patients could be recruited during exacerbations but were investigated after a stable period of at least 2 months); (3) ability to perform a six-minute walk test.
A COPD patient is considered to have acute exacerbation if there is acute deterioration in symptoms of chronic dyspnea, sputum production, or sputum purulence.
Exclusion criteria were: (1) co-existing acute pulmonary tuberculosis, pulmonary fibrosis, bronchiectasis, pneumothorax; (2) inability to perform spirometry or being physically ill or mentally incapacitate to participate; (3) receiving corticosteroids or immunosuppressive medications; (4) unstable coronary artery disease; (5) neurological disease; and (6) absence of informed consent.
The crude prevalence of COPD in India in 2016 was 4·2% [19]. Sample size required for the study was calculated using the formula.
Z2p (1-p)/d2Considering prevalence of COPD as 4% and p=0.04, Z=1.96, d=0.05 (assuming precision error of 5% and type 1 α error 5%. Sample size calculated was 60.
Altogether 172 patients were screened, of which 158 were eligible according to the inclusion and exclusion criteria. Of these, 132 patients agreed to participate in the study and considering 12 dropouts in one year follow-up, 120 subjects were included for study analysis. The medical records and discharge cards of all patients were manually reviewed. Demographic and clinical data were extracted. Demographic data included age, sex, marital status, highest form of education received (low level: illiterate and primary education; high level: secondary education and graduate). Participants were asked about their smoking habits and exposure to biomass fuel. Comorbidity was measured by the Deyo’s adapted Charlson score [20].
Severity of depression was estimated using Hamilton Depression Rating (HAM-D), and the quality of life was estimated using disease (COPD) specific St George’s Respiratory Questionnaire (SGRQ) and Generic Health related quality of life (HRQoL) SF-36 Scale. For the purpose of this study, the SGRQ % total scores were divided into quartiles as follows: Q1≤25, Q2=25-49, Q3=50-74 and Q4≥75.
Measurement of Depression
The Hamilton depression rating scale (HAM-D) is a useful way of determining a patient’s level of depression before, during and after treatment [21]. Although the HAM-D form lists 21 items, the scoring is based on the first 17. It generally takes 15-20 minutes to complete the interview and scoring the results. Eight items are scored on a 5-point scale, ranging from 0=not present to 4=severe. Nine are scored from 0-2. Depending upon the total score (range from 0 to 27), the severity of depression was classified as follows: none (0-7), mild (8-13), moderate (14-18), severe (19-22) and very severe (23-27). Patients diagnosed with depression or other psychiatric comorbidities were treated by the specialist in Dept. of Psychiatry according to the standard guidelines.
Assessments of COPD
Lung function impairment: Was assessed by spirometry after inhalation of 400 μg salbutamol using a computerised spirometer (Model vitalograph 6800; SN.PN06011Vitalograph Ltd., Ireland). Measurements followed American Thoracic Society criteria for Spiro metric standardisation and procedures [22,23].
Exercise tolerance: Six-minute walk tests (6MWTs) were performed using 25 meters walk track with two attempts conducted on the same day, at least 30 minutes apart [24]. The patient’s breathlessness was scored using modified Medical Research Council (mMRC) Dyspnea scale. The mMRC Dyspnea Scale stages five categories of breathlessness: 1 shortness of breath with strenuous exercise; 2 shortness of breath when hurrying on level ground; 3 need to stop after walking 100 meters; 4 too breathless to leave the house. Additionally, the BODE index was calculated for classification of COPD. The score comprises Body mass index (BMI), post-bronchodilator FEV1% predicted, grade of dyspnea (measured by the modified Medical Research Council dyspnea scale, MMRC) and the six-minute-walking distance [25]. The BODE index was calculated as described: for each threshold value of FEV1% predicted, distance walked in six minutes and score on the MMRC dyspnea scale [26], the patients received points ranging from 0 (lowest value) to 3 (maximal value BODE stage 1=BODE index 0-2; BODE stage 2=BODE index 3-4; BODE stage 3=BODE index 5- 6; BODE stage 4=BODE index 7-10.
Disease (COPD) specific health related quality of life (HRQoL)- SGRQ questionnaire: Burden of symptoms, physical and social functional status and impairment of quality of life were measured using the validated Hindi version of “Saint George Respiratory Questionnaire” (SGRQ) which is a self-administered disease-specific HRQoL measure, ranging from zero (indicating no impairment) to 100 [27]. Higher scores indicate a worse health status. The St. George Respiratory Questionnaire Symptoms, Impact and Activity (SGRQ_I, SGRQ_S and SGRQ_A), questionnaire assesses the patient’s experience of symptoms, the amount of distress caused by symptoms and the daily limitation of activities. It has been well validated for use in medical patients.
SF-36 generic HRQoL: According to Ware JE Jr and Sherbourne CD, [28], the SF-36 is a generic quality-of-life instrument that has two summary measures: The Physical Component Summary (PCS) and the Mental Component Summary (MCS) [29]. Scores range from zero (worst possible impairment) to 100 (good quality of health). The SF-36 is also well validated to be used for hospital patients. The SF-36 has eight scaled scores; the scores are weighted sums of the questions in each section including vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning and mental health.
Level of physical activity: The level of physical activity was subjectively assessed by using the modified Baecke questionnaire, which were administered as an interview. The Modified Baecke’s Questionnaire-physical exercise in leisure (mBQ PEL); leisure and locomotion activities (mBQ LLA); and total score (mBQ TOT), validated by Pols MA and Peeters PH, was administered to assess the patient’s usual exercise capacity subjectively [30]. It comprises 12 questions related to 3 domains: activities of daily living; sports; and leisure activities. The activities of daily living domain contain 10 questions, which were answered on a 0 to 3 scale, with 0 meaning “never does the task” and 3 meaning “always does the task”. The other domains comprise open-ended questions in which patients report the time of year when they do sports and leisure activities and the amount of time they spend on these activities. For the activities of daily living domain, the final score was calculated by adding up the points assigned to each question and dividing the result by the total number of questions in that domain; for the other domains, the final score was calculated with a code that classifies the energy expenditure level of the given activity. Finally, the three domain scores were added up, and the level of physical activity of the patient was determined. For the univariate analysis, MBQ TOT Score<4.5 was used as an indicator of impaired physical activity.
Statistical Analysis
The results are shown as mean SD unless stated otherwise. Kolmogorov-Smirnov test was performed before the data analysis to examine the data distribution of the overall sample. Continuous variables were compared using Student’s t-test for paired samples, whereas the Fisher-exact test was used for categorical data. Multivariate linear regression analysis was performed using the Modified Baecke’s Questionnaire total score (mBQ TOT) less than 4.5 as the determinant of impaired physical activity. All tests were 2-sided and p-values <0.05 were considered statistically significant. Statistical tests were performed using SPSS 21.0 (SPSS, Chicago, Illinois).
Results
Subject characteristics, pulmonary impairment, history and quality of life are depicted in [Table/Fig-1,2]. Ninety subjects (75% of the sample) had depression.
Comparison of demographic, anthropometric, clinical characteristics, and level of depression of the COPD Subjects studied expressed in frequency (% of total).
| Clinical characteristics-Category | Clinical characteristics-Subgroup | Subjects without depression (N=30) | Subjects with depression (N=90) | p-value |
|---|
| Age_group | ≤45 years | 0.0 | 1 (0.8) | 0.826 |
| 46-55 years | 14 (11.67) | 46 (38.3) |
| 56-65 years | 13 (10.83) | 31 (25.8) |
| More than 65 years | 3 (2.5) | 12 (10) |
| BODE_quartile | BODE 1-2 | 27 (22.5) | 5 (4.2) | <0.001 |
| BODE 3-4 | 3 (2.5) | 25 (20.8) |
| BODE 5-6 | 0.0 | 35 (29.2) |
| BODE 7-10 | 0.0 | 25 (20.8) |
| GOLD | 0 (Cb) | 9 (7.5) | 2 (1.7) | <0.001 |
| 1 (Mild) | 20 (16.7) | 21 (17.5) |
| 2 (Moderate) | 1(0.8) | 47 (39.2) |
| 3 (Severe) | 0.0 | 14 (11.7) |
| 4 (very severe) | 0.0 | 6 (5) |
| Mmrc | 0 | 5 (4.2) | 0 | <0.001 |
| 1 | 18 (15) | 12 (10) |
| 2 | 6 (5) | 51 (42.5) |
| 3 | 1 (0.8) | 23 (19.2) |
| 4 | 0.0 | 4 (3.3) |
| Sex | Male | 26 (21.7) | 45 (37.5) | <0.001 |
| Female | 4 (3.3) | 45 (37.5) |
| Previous hospitalisation | No Hosp. | 30 (25) | 61 (50.8) | 0.001 |
| (Hosp.) | ≥1 Hosp. | 0 | 29 (24.16) |
| Education | Primary education or less | 11 (9.2) | 53 (44.2) | 0.035 |
| More than primary education | 19 (15.8) | 37 (30.8) |
| Smoking habit | Current or previous smoker | 17 (14.2) | 55 (45.8) | 0.67 |
| Non-smoker | 13 (10.8) | 35 (29.2) |
Abbreviations: BODE: Body mass index, Airway obstruction, Dyspnea, Exercise capacity, Global initiative for chronic obstructive lung disease (GOLD), Modified medical research council (MMRC)
Comparison of demographic, anthropometric, clinical characteristics, and level of depression of the COPD Subjects expressed in Mean±Std. deviation.
| Clinical characteristics | Subjects without depression (N=30) | Subjects with depression (N-90) | p-value |
|---|
| Age | 58.1±6.43 | 57.72±7.95 | 0.794 |
| CAT | 7.03±1.9 | 19.77±8.85 | <0.001 |
| BMI | 23.38±2.80 | 22.3±2.38 | 0.064 |
| MWD6 | 338.63±59.83 | 258.27±76.6 | <0.001 |
| FEV1% | 82.46±5.53 | 62.12±16.5 | <0.001 |
| BODE | 1.1±0.31 | 3.69±1.9 | <0.001 |
| SGRQ_I | 34.47±13.3 | 46.76±17.2 | <0.001 |
| SGRQ_S | 41±17.6 | 58.73±21.4 | <0.001 |
| SGRQ_A | 25.7±8.62 | 46.24±19.63 | <0.001 |
| HAM-D | 4.03±1.86 | 17.47±5.63 | <0.001 |
| MCSSF36 | 78.57±11.9 | 48.42±17.81 | <0.001 |
| PCSSF36 | 68.53±11.9 | 47.46±10.54 | <0.001 |
| mBQPEL | 2.28±0.38 | 1.86±0.3 | <0.001 |
| mBQLLA | 2.8±0.36 | 2.07±0.36 | <0.001 |
| mBQTOT | 5.09±0.42 | 3.92±0.54 | <0.001 |
| Charlson comorbidity score | 0.87±0.5 | 1.43±0.8 | 0.001 |
| WARD-LOS | 2.47±1.77 | 5.73±2.67 | <0.001 |
Abbreviations: Combined assessment test (CAT), Body mass index (BMI), Six minute walk distance (MWD6), Percentage PREDICTED of Forced expiratory volume in 1 second (FEV1%), BODE: Body mass index, airway obstruction, dyspnea, exercise capacity, St. George Respiratory Questionnaire Symptoms, Impact and Activity (SGRQ_I , SGRQ_S and SGRQ_A), Hamilton Depression Scale (HAM-D), Short Form 36 Quality of Life Questionnaire-Physical & Mental Component Scale (PCS SF 36 & MCS SF 36), Modified Baecke’s Questionnaire-physical exercise in leisure (mBQ PEL), leisure and locomotion activities (mBQ LLA) and total score (mBQ TOT), Length of stay in ward (WARD-LOS)
Features of Depressed Subjects
The prevalence of depression was 91.83% in COPD females (n=49) and 63.38% in males (n=71). The frequency distribution of participants in terms of age, education, smoking habits and BMI was indifferent between the depressed and non-depressed groups [Table/Fig-1,2]. However, depressed subjects showed impaired physical activity, respiratory function and quality of life as demonstrated by St. George Respiratory Questionnaire, Short Form 36 Quality of Life, Modified Baecke’s Questionnaire, Precentage (%) predicted of Forced expiratory volume in one second (FEV1%), Combined Assessment Test (CAT), Length of stay in ward (WARD-LOS), Global Initiative for Chronic Obstructive Lung Disease (GOLD), BODE, and a reduced distance walked in the MWD6, which was statistically significant.
It was observed that the percentage of the depressed subjects were predominantly from GOLD Scale 2, MMRC Scale 2 and BODE Score 5-6 as 39.2%, 42.5% and 29.2% respectively. Total 29 (24%) subjects with depression had previous hospitalisation. Most (51%) of the depressed subjects were from age group 46-55 years. Among the 15 subjects over the age of 65 years, depression was prevalent in 12 subjects.
Features of Subjects with a Low Daily Physical Activity
The [Table/Fig-3] shows that subjects with a low physical activity (MBQ TOT Score <4.5) had a statistically significant (p<0.001) higher score of depression (HAM-D depression score of 19.04±5.37 vs. 7.22±3.89), a lower MWD6 (244±78 vs. 327±54.6 m), a lower Quality of life PCS SF36 (44.7±9.7), a higher pulmonary function impairment depicted by CAT (22±8.59 vs. 8.73±3.06), a higher level SGRQ_S (59.82±21.16 vs. 46.31±20.6), SGRQ_A (48.77±21 vs. 30±9.9) and higher WARD-LOS (6.52±2.29 vs. 2.59±1.74).
Demographic characteristics, anthropometric characteristics, and level of physical activity of the COPD Subjects studied. α
| Characteristics | Total | Spi+ Group (μ) | Spi- Group (μ) | p-value* |
|---|
| n=120 | n=71 | n=49 | |
| Age | 57.82±7.57 | 58.21±8.3 | 57.24±6.4 | 0.475 |
| Men/Women (n/n) | 71/49 | 33/38 | 38/11 | 0.001 |
| BMI (kg/m2) | 22.57±2.52 | 21.96±2.35 | 23.45±2.52 | 0.002 |
| Education |
| 1° Education or Less© | 64 (53.33%) | 41 (38.35%) | 23 (15%) | 0.249 |
| More than 1° Education© | 56 (46.66%) | 30 (28.3%) | 26 (18.35%) | |
| Smoking habit |
| Current/Ex-smoker© | 72 (60%) | 46(42.5%) | 26 (17.5%) | 0.201 |
| Non-smoker© | 48 (40%) | 25 (28.33%) | 23 (15.83%) | |
| BODE score | 3.04±2.1 | 4.21±1.91 | 1.35±0.48 | 0.001 |
| BODE Quartile 1 (Score 1-2)© | 32 (26.7%) | 11 | 49 (26.7%) | |
| BODE Quartile 2 (Score 3-4)© | 28 (23.3%) | 35(9.2%) | 0 | |
| BODE Quartile 3 (Score 5-6)© | 35 (29.2%) | 13(29.2%) | 0 | |
| BODE Quartile 4 (Score 7-10)© | 25 (20.8%) | 12(20.8%) | 0 | |
| WARD_LOS | 4.91±2.84 | 6.52±2.29 | 2.59±1.74 | <0.001 |
| HAM-D | 14.22±7.56 | 19.04±5.37 | 7.22±3.89 | <0.001 |
| PCS SF-36 | 52.72±14.2 | 44.77±9.7 | 64.24±11.58 | <0.001 |
| MCS SF-36 | 55.96±21.1 | 44.34±16.97 | 72.8±13.84 | <0.001 |
| SGRQ_A | 41.11±19.6 | 48.77±21 | 30±9.85 | <0.001 |
| SGRQ_S | 54.30±21.87 | 59.82±21.16 | 46.31±20.6 | 0.001 |
| SGRQ_I | 43.68±17.11 | 47.82±17.67 | 37.69±14.4 | 0.001 |
| FEV1_litres | 2.01±0.53 | 1.72±0.49 | 2.4±0.26 | <0.001 |
| MWD6 (metre) | 278.36±80 | 244±78 | 327±54.6 | <0.001 |
| CAT score | 16.58±9.49 | 22±8.59 | 8.73±3.06 | <0.001 |
| mBQ_PEL | 1.96±0.37 | 1.76±0.231 | 2.26±0.34 | <0.001 |
| mBQ_LLA | 2.25±0.48 | 1.94±0.25 | 2.7±0.37 | <0.001 |
| mBQ_TOT | 4.22±0.72 | 3.7±0.36 | 4.9±0.38 | <0.001 |
αValues expressed as mean±Std. Dev; except where otherwise indicated; ©Values expressed as n (total %); *Mann-Whitney test or unpaired t-test; μSPI+_group (MBQ TOT<4.5), SPI-_group (MBQ TOT>4.5
Abbreviations: No severe physical activity (SPI−), Severe physical activity (SPI+), Body mass Index (BMI), Body mass index; airway Obstruction; Dyspnea; Exercise capacity (BODE), Length of stay in ward (WARD-LOS), Hamilton Depression Scale (HAM-D), ), Short Form 36 Quality of Life Questionnaire-Physical & Mental Component Scale (PCS SF 36 & MCS SF 36), ), St. George Respiratory Questionnaire Symptoms, Impact and Activity (SGRQ_I , SGRQ_S and SGRQ_A), Forced expiratory volume in 1 second (FEV1 litres), Six Minute Walk Distance (MWD6), Combined Assessment Test (CAT), Modified Baecke’s Questionnaire-physical exercise in leisure (mBQ PEL), leisure and locomotion activities (mBQ LLA) and total score (mBQ TOT)
The [Table/Fig-4] depicts that in the multivariate analysis, CAT Score, BMI, MWD6, depression, quality of life (PCS SF36) and length of stay in ward were predictors of physical impairment.
Predictors of impaired physical activity (MBQ TOT<4.5) in a Multivariate linear regression analysis.
| Std. | Regression | Coefficientsa | | |
|---|
| Predictive parameters | All variables in the same equation | Individual variables in the equation |
|---|
| Exp (B) | p-value | R square change | Exp (B) | p-value |
|---|
| CAT | 1.63 | 0.003 | 0.661 | 0.711 | 0.001 |
| BMI | 1.946 | 0.004 | 0.113 | 1.288 | 0.001 |
| MWD6 | 1.020 | 0.014 | 0.346 | 1.017 | 0.001 |
| FEV1 percentage | 3.948 | 0.220 | | | |
| SGRQ_I | 0.968 | 0.129 | | | |
| SGRQ_S | 1.015 | 0.266 | | | |
| SGRQ_A | 0.945 | 0.099 | | | |
| HAM-D | 0.541 | 0.022 | 0.772 | 0.591 | 0.001 |
| MCSSF36 | 1.061 | 0.136 | | | |
| PCSSF36 | 1.325 | 0.001 | 0.659 | 1.303 | 0.001 |
| WARDLOS | 0.297 | 0.003 | 0.625 | 0.374 | 0.001 |
A-BooStrapping Results
Abbreviations: Modified baecke’s Questionnaire For Physical Activity- Total Score (Mbq Tot), Combined Assessment Test (Cat), Body Mass Index (Bmi), Six Minute Walk Distance (Mwd6), % Predicted Of Forced Expiratory Volume In 1 Second (Fev1%), St. George Respiratory Questionnaire Symptoms, Impact And Activity (Sgrq_i , Sgrq_s And Sgrq_a), Hamilton Depression Scale (Ham-D), Short Form 36 Quality Of Life Questionnaire-Physical & Mental Component Scale (Pcs Sf 36 & Mcs Sf 36), Length Of Stay In Ward (Ward-Los)
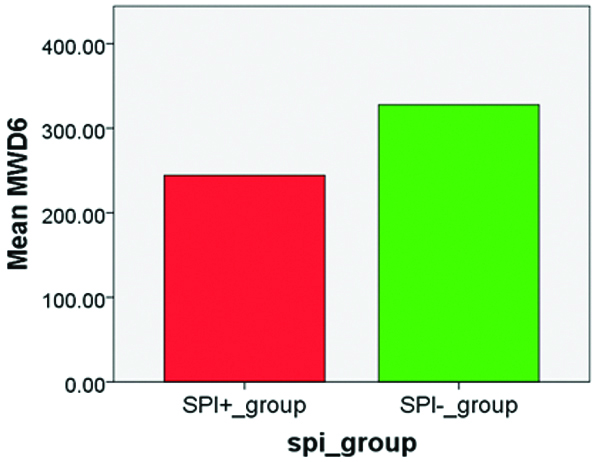
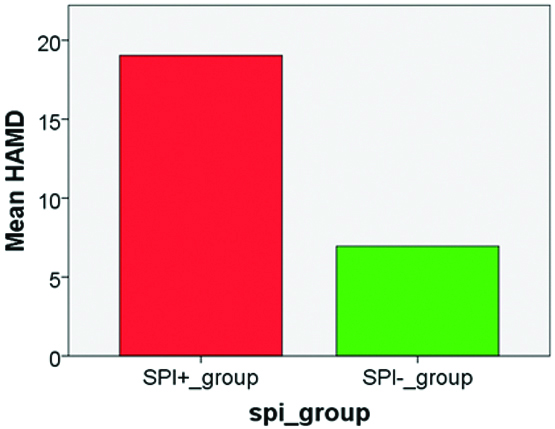
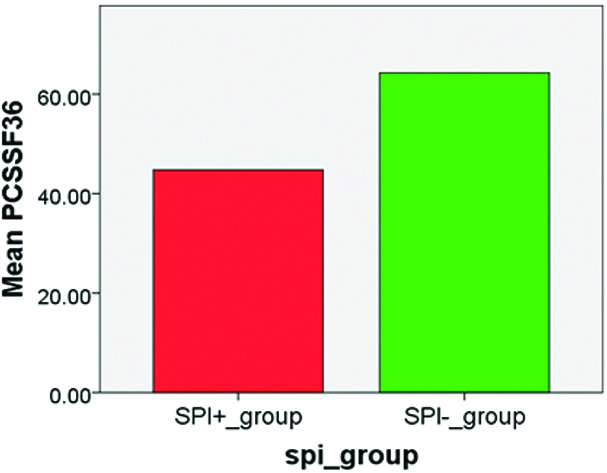
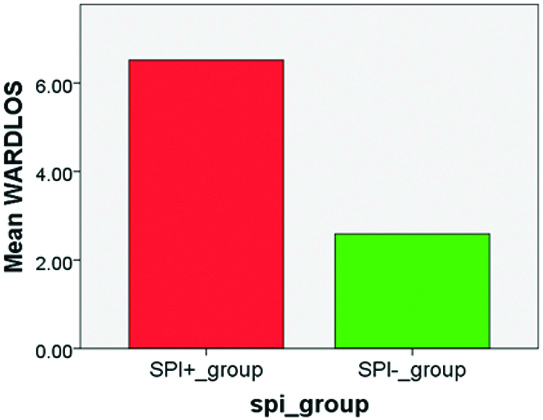
Differences in the mean scores of MWD6, HAM D, PCS SF36, WARD LOS on the X Axis and between Severely physically impaired (SPI +) subjects (n=71) and Non-Severely physically impaired (SPI -) subjects (n=49) on the Y Axis is depicted in [Table/Fig-5,6,7 and 8].
The distance walked (expressed in meters) during the 6-min walk test (MWD6).
Hamilton Depression Scale (HAM-D).
Short Form 36 Quality of Life Questionnaire-Physical Component Scale (PCS SF 36).
Length of stay in ward (WARD LOS).
The [Table/Fig-5,6,7 and 8] Differences in mean scores between Severely physically impaired (SPI+) subjects (n=71) and Non-Severely physically impaired (SPI-) subjects (n=49) on Y axis. The X Axis was as follows in the figures.
Discussion
To the best of authors knowledge, this is one of initial studies carried out with subjects of COPD; specifically designed to explore the influence of depression and physical activity levels on each other and on socio-demographic profile, symptoms, disease severity, stay in ward and quality of life, all parameters simultaneously in Indian population. Depressed subjects displayed reduced physical exercise in leisure (mBQ PEL), leisure and locomotion activities daily (mBQ LLA) and total physical activities (mBQ TOT), as demonstrated by the modified Baecke questionnaire and a reduced distance walked in the MWD6 despite similar levels of age, education, smoking status and BMI compared to non-depressed COPD patients. Moreover, both depressed and physically impaired subjects assessed independently had higher level of shortness of breath (SGRQ, FEV1 litres), COPD Severity (BODE), increase ward stay, and a worse quality of life (SF 36) compared to the other group (p<0.01).
Like the ECLIPSE study, this study also predicted worse quality of life and a higher dyspnea levels in depressed individuals, predominantly in females [31]. Also, the present study correlates with Al-shair K et al., who, in a cohort of 122 patients with stable COPD, found no significant difference between subjects with and without depression, in muscle wasting, as was with BMI in the present study [32]. Also, there was a significant difference in exercise capacity depicted by the MWD6 between subjects with and without depression. The high prevalence of depression in the present study also correlates with some previous Indian studies [33,34]. The correlation between FEV1 and MWD6 with the levels of depression also matches with findings in the ECLIPSE study [15,31]. It is noteworthy that subjects with good physical activity levels have an improved respiratory disability as compared to physically impaired COPD subjects [9]. Because of limited enrollment of COPD subjects, age was not different between depressed or physically impaired subjects with the other groups. This finding was in contrast with the findings of the ECLIPSE study. Our study imparts knowledge like the cross-sectional study of Watz H et al., where depression itself was not an independent factor correlating with levels of physical activity [35]. In the present study, low BMI was a risk factor for impaired physical activity, in contrast to the general population [36] due to the pathophysiological process of muscle wasting in subjects of COPD, which independently acts in reducing the daily physical activity level. Similarly, though COPD influences impairment of physical activity levels, both BMI and the grade of dyspnea have independent influence on the physical activity levels. Depression itself is associated with poor perception in the quality of life and increased level of shortness of breath [2,31]. Hence, there lies a complexity in explaining the reasons for impairment of physical activity in COPD patients, influenced by diverse factors, such as the functional impact of the respiratory disease and the perceived level of symptoms and quality of life impairment. As discussed above, it can be hypothesised that depression is more of an effect rather than the cause of impaired daily physical activity.
The multivariate analysis in the present study found six parameters as predictors of impaired physical activity: CAT Score, BMI, MWD6, Depression (HAM D), quality of life (PCS SF36) and length of stay in ward. In previously conducted studies, decreased physical activity was associated with a decline in lung function, and muscle weakness in COPD subjects [37,38]. In contrast to our study, Van Remoortel H et al., demonstrated that comorbidities were more strongly associated than FEV1 with the physical inactivity [39]. However, there is paucity of prospective study data that has successfully found an association between physical activity level and the presence of comorbidities longitudinally in COPD subjects. Watz H et al., has depicted weak association between levels of physical activity and lung function [40]. However, COPD patients with dynamic hyperinflation have an inverse relationship with the physical activity levels [41], correlated more strongly with the degree of dyspnea [42]. It is interesting to note that previously authors have also demonstrated an association of physical activity with the self-efficacy, defined as an individual’s belief in their ability to be successful in something [43,44]. Also, Lee SH et al., identified clinical factors associated with low levels of physical activity like our study [45].
The study has few strengths as there is paucity of data which analyses physical activity patterns and predictors of low-level physical activity in subjects with COPD. Also, exploring the influence of depression and physical activity levels on each other and on sociodemographic profile, symptoms, disease severity, stay in ward and quality of life, all parameters simultaneously merit the study in this part of world.
Limitation
The authors were unable to explain the causal relationship between low physical activity and the identified risk factors due to cross-sectional study design. Also, there were only two categories of educational level i.e., 1) primary education or less and 2) more than primary education. Lastly, the study also did not consider the food intake and socio-economic status, parameters that could have helped us in the evaluation of the reason for a potential difference in BMI between depressed and non-depressed subjects.
Conclusion
This study revealed that depression, quality of life and daily physical activity are important entities that should be assessed during the management of COPD subjects. Determinants of impaired physical activity in COPD subjects is depicted by symptoms score (CAT Score), exercise capacity (MWD6), nutritional status (BMI), depression (HAM-D), quality of life (PCS SF36) and length of stay in ward. Complete assessment of all these parameters also merits the present study.
Abbreviations: BODE: Body mass index, Airway obstruction, Dyspnea, Exercise capacity, Global initiative for chronic obstructive lung disease (GOLD), Modified medical research council (MMRC)
Abbreviations: Combined assessment test (CAT), Body mass index (BMI), Six minute walk distance (MWD6), Percentage PREDICTED of Forced expiratory volume in 1 second (FEV1%), BODE: Body mass index, airway obstruction, dyspnea, exercise capacity, St. George Respiratory Questionnaire Symptoms, Impact and Activity (SGRQ_I , SGRQ_S and SGRQ_A), Hamilton Depression Scale (HAM-D), Short Form 36 Quality of Life Questionnaire-Physical & Mental Component Scale (PCS SF 36 & MCS SF 36), Modified Baecke’s Questionnaire-physical exercise in leisure (mBQ PEL), leisure and locomotion activities (mBQ LLA) and total score (mBQ TOT), Length of stay in ward (WARD-LOS)