Introduction
Dengue is one of the most common arthropod-borne viral disease in the tropical and subtropical regions of the world. The disease is a burden in terms of morbidity, mortality and economic aspect. There is no specific drug for dengue infection yet and the availability of a vaccine is limited in most of the Indonesia’s endemic areas. In endemic areas, most of dengue fever cases clinically resemble other diseases such as malaria, typhus, or just flu-like syndrome. Early verification of suspected dengue outbreaks is very important, allowing better public health response that leads to a proper clinical care. Unfortunately, the use of dengue Rapid Diagnostic Tests (RDTs) is still rare at first level of the health care provider in Indonesia, where the country has the policy of a gradual health service supported by the National Health Insurance (NHI).
Aim
The study measured the usefulness of dengue RDT at the Primary Health Centre (PHC) in endemic areas.
Materials and Methods
DENV NS1 antigen test kit (NS1 RDT) was used to detect dengue fever for all acute fever patients at three sites of PHC which had high dengue case history. Dengue fever 2017 report was used as comparative data for the analysis. The collected data were analysed using an independent t-test.
Results
A total of 237 blood samples from patients with acute fever were collected from Sukajadi, Neglasari and Padasuka PHC. Fifty-one samples out of 237 (21.5%) were positive with the DENV NS1 antigen test. The number of reported dengue case before and after the implementation of DENV NS1 antigen test increased significantly (p<0.05) for each PHC; Sukajadi (p=0.01), Neglasari (p=0.01) and Padasuka (p=0.03).
Conclusion
NS1 RDT effectively increased the diagnosed and reported cases when used in the PHCs. Hence, we strongly suggest that government should make a policy that supports the use of NS1 RDT at the PHCs for the early detection of dengue infection, since those PHCs are the spearheads in the implementation of health care programs in Indonesia.
Introduction
Dengue fever is found in tropical and subtropical regions. Data from all over the world shows Asia occupying first place in the number of dengue sufferers every year. Meanwhile, from 1968 to 2009, World Health Organisation (WHO) recorded Indonesia as the country with the highest Dengue Haemorrhagic Fever (DHF) cases in Southeast Asia [1].
Every year, about 400 million people are infected with dengue virus, 100 million become dengue and DHF require hospitalisation. Every year 21,000 deaths attributed to dengue are detected and in children, the death rate can be as high as 20% if the diagnosis is not immediately enforced and appropriate treatment is not given in time. The availability of a vaccine for dengue is very limited, until now [2].
Infected dengue patients generally present with a range of clinical signs and symptoms that vary according to age and severity [3]. The disease is characterised by an abrupt onset of high fever, accompanied by a headache and myalgia very similar to a flu-like syndrome. These symptoms can be debilitating hence the nickname ‘break bone fever’. In the past, dengue infection was graded according to four levels. Grades I-III patients have spontaneous bleeding from the skin, nose, gums and circulatory failure or hypotension. Grade IV is more severe, patients are moribund, with no detectable pulse or blood pressure. Dengue Shock Syndrome (DSS) refers to patients with grade III or IV [4]. In the recent criteria, Dengue is classified into five groups: DF (Dengue fever), DHF I, DHF II, DHF III, DHF IV [Table/Fig-1] [5].
WHO classification of dengue infections and grading of severity of DHF.
| DF/DHF | Grade | Signs and Symptoms | Laboratory finding |
|---|
| DF | | Fever with two of the following:
Headache. Retro-orbital pain. Myalgia. Arthralgia/bone pain. Rash. Haemorrhagic manifestations. No evidence of plasma leakage.
| Leucopenia (WBC≤5000 cells/mm3). Thrombocytopenia (Platelet count <150,000 cells/mm3) Rising haematocrit (5%-10%) No evidence of plasma loss
|
| DHF | I | Fever and haemorrhagic manifestation (positive tourniquet test) and evidence of plasma leakage | Thrombocytopenia (Platelet count <100,000 cells/mm3) HCT ≥20%
|
| DHF | II | As in Grade I plus spontaneous bleeding. | Thrombocytopenia (Platelet count <100,000 cells/mm3) HCT ≥20%
|
| DHF* | III | As in Grade I or II plus circulatory failure (weak pulse, narrow pulse pressure ≤20 mmHg, hypotension, restlessness). | Thrombocytopenia (Platelet count <100,000 cells/mm3) HCT ≥20%
|
| DHF* | IV | As in Grade III plus profound shock with undetectable BP and pulse | Thrombocytopenia (Platelet count <100,000 cells/mm3) HCT ≥20%
|
* DHF III and IV are DSS
Dengue fever typically resembles flu-like symptoms, typhus or malaria in the endemic country. Thus, the identification of dengue fever is not easy since the flu-like symptoms resemble so many other diseases. Some tests such as ELISA and PCR can confirm a primary infection of dengue fever [6]. A positive ELISA test show IgM greater than or equal to 40 units or IgG greater than or equal to 100 units [7].
Early verification of suspected dengue outbreaks allow for early public health response, leading to the initiation of appropriate clinical care [3]. A previous study showed that dengue virus was responsible for a significant percentage of Acute Febrile Illnesses (AFI) in an adult and children population in West Java, Indonesia. A wide range of clinical severity was observed with most infections resulting in asymptomatic disease [7]. As the clinical presentation of dengue is nonspecific, simple RDTs provide opportunities for point-of-care diagnosis [8].
The diagnosis of dengue can be done by virus isolation, by serological tests, or by molecular methods. Acute infection with dengue virus is confirmed when the virus is isolated from serum or autopsy tissue specimens, or the specific dengue virus genome is identified by Reverse Transcription-Polymerase Chain Reaction (RT-PCR) from serum or plasma, cerebrospinal fluid, or autopsy tissue specimens during an AFI [9]. However, diagnosing dengue remains a major challenge in some developing countries, including Indonesia, due to the complex sample processing and the need for specialised laboratories for confirming the diagnosis. In Indonesia, the annual reported dengue cases are usually based on the results from hospital instead of from Primary Health Centres (PHC, also called puskesmas). PHCs can not diagnose the patients with dengue fever or DHF due to the lack of laboratory facilities or NS1 RDT [10]. A definitive laboratory test such as rapid point-of-care test is advantageous, especially in the primary care setting. Among several techniques available for dengue diagnosis, the immunochromatographic rapid tests have been increasingly used in some countries to detect dengue virus (DENV) [11]. For example, ordering a DENV NS1 antigen assay (NS1 RDT) within the first week of symptom onset is one of the initial investigations recommended by the Ministry of Health Singapore for dengue infections [12], because RDTs based on DENV NS1 antigen testing can be conducted fairly rapidly, and at a lower cost [13]. A report from Vietnam even described a novel approach where dengue serotyping by RT-PCR could be done using the RNA recovered from NS1 RDT, making RDT at the primary care a great prospect for the collection of epidemiological data and public health interventions [14].
DENV RDT can have drawbacks. A study found that DENV RDT had low sensitivity for the diagnosis of dengue infection [15]. In that study, DENV RDT did not increase the accuracy of DENV diagnosis and was not helpful in deciding which children required critical care admission. Nonetheless, other groups proved that RDTs were good diagnostic tools [16,17]. We are of the opinion that RDT would be important in confirming dengue cases in the resource-limited regions in Indonesia and thus allowing a rapid and more-focused outbreak response, along with the simultaneously prevention methods such as community outreach, mosquito prevention/control, and clinician awareness.
Unfortunately, the use of dengue RDTs is still rare at first level of health care provider. This is especially important when there is a National Health Insurance (NHI) policy on gradual health service in Indonesia which had been running since 1st January 2014. The NHI was program built which is based on universal coverage system with the aim that all people could get access to healthcare services without cost prohibition [18]. Inside the program, a health care referral system is implemented in phases based on the medical needs. With this gradual referral system, the participants should visit the primary health facilities first to get the reference for the healthcare services. The secondary or tertiary care can only be given if the participants have the reference from PHC [19]. Thus, the first level of health care provides an important role as the main gate of patients with the hope that most of the health problems and services needed are solved there. PHC as the first level of health facility which has more strategic role and advantages compared to family doctors and private clinics in supporting the implementation of NHI [20]. Therefore, we conducted this research to assess the expediency of dengue RDT at the PHCs in reducing the burden of dengue in endemic areas.
Materials and Methods
This was a cross-sectional study, conducted during the period of March to September 2018. The protocol of this study was approved by the Medical Research Ethics Committee of Faculty of Medicine Universitas Padjajaran, Bandung Indonesia with approval number 071711.
Study Area
The study was conducted in Bandung City. It is the largest city in West Java Province (southern part of Java Island), as well as being the capital of the province. The city of Bandung is the most populous city in West Java Province. In 2017, the population reached 2,404,589 people with an area of 167.67 km2 and a population distribution of 14,341 people/km2. The area is divided into 30 sub-districts and 151 villages [21]. Sixty two PHCs function to deal with the health problems of Bandung residents. Each PHC covers several villages as working areas [22]. Bandung city area always has problem with dengue throughout the year, reaching 3134 cases in 2014, and it is known as the city with the highest case of dengue fever in West Java province [22,23].
Sampling Sites
The samples were collected from Neglasari PHC, Padasuka PHC, and Sukajadi PHC, respectively, which have been reported as the PHCs with the highest dengue incidents in Bandung City. The working area of the Neglasari PHC covered for Neglasari, Sukaluyu, Cigadung and Cihaurgeulis village. Padasuka PHC covered for Padasuka, Cicadas, Sukamaju and Cikutra village. While Sukajadi PHC covered for Cipedes, Pasteur, and Sukabungah village.
Surveillance Procedures
We included all outpatients who had a fever of at least 37.8°C, 1-4 days, of all age categories, with or without taking antipyretics and were willing to cooperate by filling the informed consent. Patients with a history of chronic illnesses, such as diabetes mellitus, chronic liver disease, chronic kidney disease, and human immunodeficiency syndrome were excluded. We recorded the name, address, age, and gender of each patient with AFI. The analysis was done in a blinded way.
Laboratory Procedures
The blood samples from suspected dengue patients were taken by the health care officers in the acute phase (within 1-4 days of illness) to examine the haemoglobin level, leucocytes, haematocrit and thrombocytes. DENV NS1 antigen test (NS1 RDT) was done as an additional check and was performed according to the manufacturer’s instructions of the InBios NS1 rapid test from USA. The Inbios NS1 RDT was chosen after consideration of its published sensitivity, false positive and false negative number compared to other NS1 RDTs.
Dengue Secondary Data
Dengue data in 2017 were taken from Padasuka, Neglasari and Sukajadi PHCs. The data were based on clinical and laboratory examinations such as the number of leucocytes, hematocrit, thrombocytes and hemoglobin level. These data were used for comparing the data before and after NS1 implementation.
Statistical Analysis
The collected data was analysed using an independent t-test with RStudio Desktop version 1.1.463 to test the significance of NS1 RDT implementation at the PHC.
Results
There were 239 patients with acute fever who reported to the Padasuka, Neglasari and Sukajadi primary health centres, but two patients refused to join the research. A total of 237 blood samples from patients were collected, from March until September 2018. Fifty-one samples out of 237 were positive with the rapid NS1 antigen test. Padasuka PHC had the highest number for the test as shown in [Table/Fig-2]. The percentage of NS1 positive for Padasuka PHC, Neglasari PHC, and Sukajadi PHC are 49.02%, 29.41% and 21.57% respectively.
Positive dengue case at each PHC from March-September in 2018.
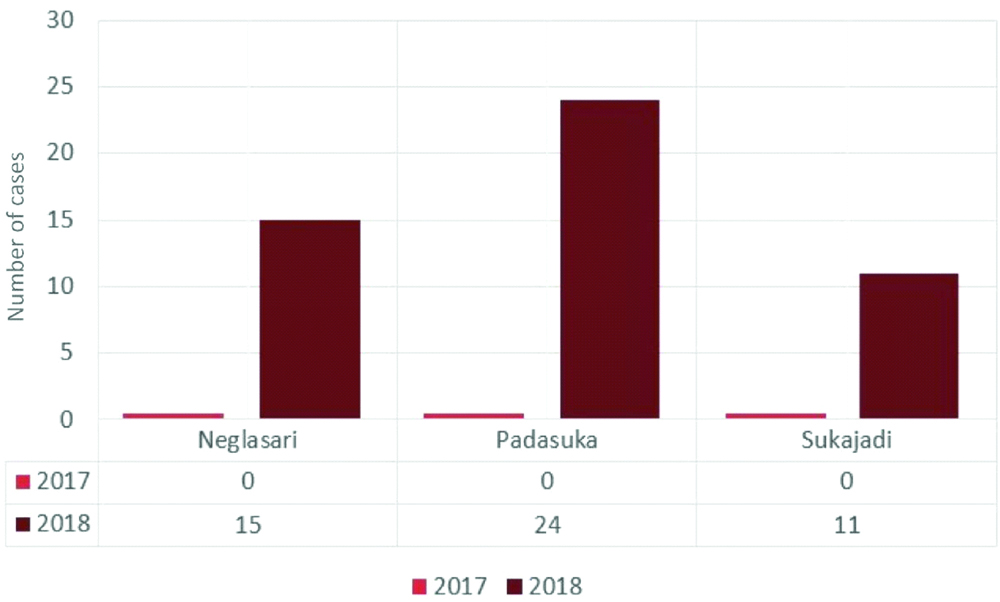
[Table/Fig-2] shows that there were no reports of dengue cases at each PHC in the year 2017, as they heavily relied on the results of routine blood analysis after day 3 of fever and never used DENV NS1 antigen test. In contrast, after implementation of the NS1 RDT in 2018, we began to see the increasing number of reported cases in these aforementioned PHCs. Therefore, the number of dengue cases comparison between year 2017 and 2018 showed significant value (p<0.05) for each PHC; Sukajadi (p=0.01), Neglasari (p=0.01) and Padasuka (p=0.03). It is to be noted that we found no significant differences on the dengue secondary data of before and after the implementation of NS1 RDT.
Discussion
This study found that the implementation of DENV NS1 antigen test (NS1 RDT) was crucial for early dengue detection in patients with acute fever at the PHCs and RDT gave significant impact of reported cases number. The NS1 test could detect dengue infection in patients with acute fever (1-4 days) from this study, and this was in accordance with other study which also showed that DENV NS1 antigen test showed the highest positivity on days 1-3 [24,25].
We used strict criteria of fever (for example, 1-4 days of fever) in recruiting the patients, however, our study detected dengue for below 50% of the fever cases. As the previous study by Pal S et al., [26] using InBios NS1 RDT showed 98.8% sensitivity for primary infection and 83.5% sensitivity for secondary infections, we ruled out the secondary dengue infection for insensitivity of the RDT in this study.
Kosasih H et al., reported that serotypes DENV-1, DENV-2, DENV-3 and DENV-4 were dominant in West Java, where Bandung City resided [23]. Moreover, the study by Pal S et al., that used retrospective samples from South America showed that the sensitivity of the RDTs ranged from 71.9%-79.1% while the sensitivity of the ELISAs varied between 85.6-95.9%, using virus isolation as the reference method [26]. It is to be noted that we did not perform virus isolation as the reference method in this study. Therefore, our lower number of dengue detection cannot be compared directly to the theoretical sensitivity of the RDT used, as the percentage of detection in this study was based on the number of fever cases.
RDTs are simple to perform and require little laboratory infrastructure. Therefore, they are suitable for use in resource-limited settings, by lesser-trained health workers or laboratory staff. However, many RDTs must be stored in a dry environment and at cool temperatures, possibly also requiring the use of cold chain (2°C to 8°C) [27,28]. This has been shown to be somewhat problematic when the PHC lack the facility or when there were power outage, possibly resulted in damaged kit. This possibility was kept minimal in our study.
Our study is the first of its kind that implemented NS1 RDT and assessed its use at the PHC in Indonesia. Dengue cases should be handled based on the region of outbreak, by early diagnosis and prompt treatment [25] which is very effective approach in controlling infectious diseases [28]. RDT is suitable and important for early diagnosis. Limited access to health services and limited ability of doctors to enforce early diagnosis of dengue cases contribute to the time delay for patients before they start to seek for treatment. In Indonesia the sufferers try several drugs before or right after the first visit to the doctor. If the illness gets worse, they visit the doctor who had treated the undiagnosed illness the first time. In some cases patients are then referred to higher centres for further examination [29]. As a result of the time delay in diagnosis, a person who has a dengue virus in his body becomes a very effective source (foci) of the disease outbreak [30]. When a case is detected and promptly treated, it cannot act as a source of further infection and thus will prevents the occurrence of an outbreak in the region. This is an active strategy to prevent and control dengue.
Recently, the NHI system in Indonesia introduced a policy that decentralised health services from hospitals or advanced health facilities. Within the new policy whose goal is to improve services for the participants of NHI, medical services must be carried out gradually according to medical needs and findings with the PHC or puskesmas as the first door of Indonesian healthcare system.
Being the first door, the quality of primary health facilities is crucial and affects the success of future NHI programs. The lack of good facility in the PHC had resulted in the phenomenon of hospital being overcrowded and delay in dispersing treatment, especially when the demand for health services increased as the community was more supported by the NHI. This is what the government wants to eliminate and avoid in the future [31]. A study had proved that primary care helps in preventing illness and death. The PHC, under study, was associated with a more equitable distribution of health service in populations [32].
Limitation
The long distance of transport for blood samples might have increased the risk of false negative due to sample lysis. It is also to be noted that we did not perform further confirmation by the nucleic acid testing for dengue nor for other causes of fevers like Chikungunya, Zika or Rickettsia. Interestingly, we found that the adherence of doctors and PHC officers to carry out or enforce the serial routine blood tests to diagnose dengue worth further investigation, especially in the lack of RDT or laboratory facility.
Conclusion
The use of dengue RDT at government PHC can prevent dengue from becoming more severe, reducing the number of hospital admissions, reducing the cost of dengue surveillance and eventually can cut the economic burden of the disease. Therefore, it is strongly suggested that the government should make a policy which supports the use of NS1 RDTs at the PHC for early detection of dengue infection; as the primary health care centres are the spearheads of the government program in improving Indonesian public health.
* DHF III and IV are DSS
[1]. CDC. Why is dengue a global issue? Center For Diseases Control and Prevention [Internet]. Available from: https://www.cdc.gov/dengue/training/cme/ccm/page51440.htm [Google Scholar]
[2]. Ayukekbong JA, Oyero OG, Nnukwu SE, Mesumbe HN, Fobisong CN, Value of routine dengue diagnosis in endemic countriesWorld J Virol 2017 6(1):9-16.10.5501/wjv.v6.i1.928239567 [Google Scholar] [CrossRef] [PubMed]
[3]. (TDR) WHO and the SP for R and T in TD. Dengue guidelines for diagnosis, treatment, prevention and control: new edition [Internet]. WHO Press; 2009. Available from: https://www.who.int/rpc/guidelines/9789241547871/en/ [Google Scholar]
[4]. McBride WJ, Bielefeldt-Ohmann H, Dengue viral infections; pathogenesis and epidemiologyMicrobes and infection 2000 2(9):1041-50.10.1016/S1286-4579(00)01258-2 [Google Scholar] [CrossRef]
[5]. Megawati D, Masyeni S, Yohan B, Lestarini A, Hayati RF, Meutiawati F, Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue virusesPLOS Neglected Tropical Diseases 2017 11(5):e000548310.1371/journal.pntd.000548328531223 [Google Scholar] [CrossRef] [PubMed]
[6]. Shan C, Ortiz DA, Yang Y, Wong SJ, Kramer LD, Shi PY, Evaluation of a novel reporter virus neutralization test for serological diagnosis of Zika and Dengue virus infectionJ Clin Microbiol 2017 55(10):3028-36.10.1128/JCM.00975-1728768729 [Google Scholar] [CrossRef] [PubMed]
[7]. Souf S, Recent advances in diagnostic testing for viral infectionsBioscience Horizons: The International Journal of Student Research 2016 9:hzw010-hzw. [Google Scholar]
[8]. Blacksell SD, Commercial dengue rapid diagnostic tests for point-of-care application: Recent evaluations and future needs?Journal of Biomedicine & Biotechnology 2012 2012:15196710.1155/2012/15196722654479 [Google Scholar] [CrossRef] [PubMed]
[9]. CDC. No Title Laboratory Guidance and Diagnostic Testing United States: Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Vector-Borne Diseases (DVBD) [Internet]. Available from: https://www.cdc.gov/dengue/clinicallab/laboratory.html [Google Scholar]
[10]. Wahyono TYM, Nealon J, Beucher S, Prayitno A, Moureau A, Nawawi S, Indonesian dengue burden estimates: Review of evidence by an expert panelEpidemiology and Infection 2017 145(11):2324-29.10.1017/S095026881700103028545598 [Google Scholar] [CrossRef] [PubMed]
[11]. Prado PS, Almeida Júnior JTD, Abreu LTd, Silva CG, Souza LdC, Gomes MC, Validation and reliability of the rapid diagnostic test ‘SD Bioeasy Dengue Duo’ for dengue diagnosis in Brazil: A phase III studyMemorias do Instituto Oswaldo Cruz 2018 113(8):e170433-e.10.1590/0074-0276017043329947711 [Google Scholar] [CrossRef] [PubMed]
[12]. Mark N, Chung W, Revisiting the approach to dengue: The primary care perspectiveThe Singapore Family Physician 2015 41(2):65-73. [Google Scholar]
[13]. Chan HBY, How CH, Ng CWM, Definitive tests for dengue fever: When and which should I use?Singapore Medical Journal 2017 58(11):632-35.10.11622/smedj.201710029167907 [Google Scholar] [CrossRef] [PubMed]
[14]. Castonguay-Vanier J, Klitting R, Sengvilaipaseuth O, Piorkowski G, Baronti C, Sibounheuang B, Molecular epidemiology of dengue viruses in three provinces of Lao PDR, 2006-2010PLOS Neglected Tropical Diseases 2018 12(1):e000620310.1371/journal.pntd.000620329377886 [Google Scholar] [CrossRef] [PubMed]
[15]. Carter MJ, Emary KR, Moore CE, Parry CM, Sona S, Putchhat H, Rapid diagnostic tests for dengue virus infection in febrile Cambodian children: diagnostic accuracy and incorporation into diagnostic algorithmsPLoS Neglected Tropical Diseases 2015 9(2):e0003424-e.10.1371/journal.pntd.000342425710684 [Google Scholar] [CrossRef] [PubMed]
[16]. Shukla MK, Singh N, Sharma RK, Barde PV, Utility of dengue NS1 antigen rapid diagnostic test for use in difficult to reach areas and its comparison with dengue NS1 ELISA and qRT-PCRJ Med Virol 2017 89(7):1146-50.10.1002/jmv.2476428042883 [Google Scholar] [CrossRef] [PubMed]
[17]. Hunsperger EA, Sharp TM, Lalita P, Tikomaidraubuta K, Cardoso YR, Naivalu T, Use of a rapid test for diagnosis of dengue during suspected dengue outbreaks in resource-limited regionsJournal of Clinical Microbiology 2016 54(8):2090-95.10.1128/JCM.00521-1627225409 [Google Scholar] [CrossRef] [PubMed]
[18]. WHO. Health System. Questions and Answers on Universal Health Coverage: WHO; 2011 [Internet]. Available from: https://www.who.int/healthsystems/topics/financing/uhc_qa/en/ [Google Scholar]
[19]. WHO. WHO Report: The World Health Report 2008-primary Health Care (Now More Than Ever): WHO 2008 [Internet]. Available from: https://www.who.int/whr/2008/en/ [Google Scholar]
[20]. Reduction N team for the acceleration of poverty. The Road to National Health Insurance (JKN) [Internet]. Available from: http://www.tnp2k.go.id/images/uploads/downloads/FINAL_JKN_road to national health insurance.pdf [Google Scholar]
[21]. Somantri L, The spatial interaction of bandung citizensIndonesian Journal of Geography 2013 45(2):9 [Google Scholar]
[22]. Ministry of Health Republic of Indonesia. Indonesia Health Profile. [Internet]. Available from: http://www.depkes.go.id/resources/download/pusdatin/profil-kesehatan-indonesia/Indonesia Health Profile 2014.pdf [Google Scholar]
[23]. Kosasih H, Alisjahbana B, Nurhayati de Mast Q, Rudiman IF, Widjaja S, The epidemiology, virology and clinical findings of dengue virus infections in a cohort of indonesian adults in western javaPLOS Neglected Tropical Diseases 2016 10(2):e0004390.2410.1371/journal.pntd.000439026872216 [Google Scholar] [CrossRef] [PubMed]
[24]. Hermann LL, Thaisomboonsuk B, Poolpanichupatam Y, Jarman RG, Kalayanarooj S, Nisalak A, Evaluation of a dengue NS1 antigen detection assay sensitivity and specificity for the diagnosis of acute dengue virus infectionPLoS Neglected Tropical Diseases 2014 8(10):e3193-e.10.1371/journal.pntd.000319325275493 [Google Scholar] [CrossRef] [PubMed]
[25]. Solanke VN, Karmarkar MG, Mehta PR, Early dengue diagnosis: Role of rapid NS1 antigen, NS1 early ELISA, and PCR assayTrop J Med Res 2015 18(2):95-9.10.4103/1119-0388.158402 [Google Scholar] [CrossRef]
[26]. Pal S, Dauner AL, Mitra I, Forshey BM, Garcia P, Morrison AC, Evaluation of dengue NS1 antigen rapid tests and ELISA kits using clinical samplesPLoS One 2014 9(11):e11341110.1371/journal.pone.011341125412170 [Google Scholar] [CrossRef] [PubMed]
[27]. Casenghi M, Kosack C, Li R, Bastard M, Ford N, NS1 antigen detecting assays for diagnosing acute dengue infection in people living in or returning from endemic countriesCochrane Database of Systematic Reviews 2014 (6)10.1002/14651858.CD011155 [Google Scholar] [CrossRef]
[28]. Ameli J, Communicable diseases and outbreak controlTurkish Journal of Emergency Medicine 2015 15(Suppl 1):20-26. [Google Scholar]
[29]. Glass BD, Counterfeit drugs and medical devices in developing countriesRes Rep Trop Med 2014 20(5):11-22.10.2147/RRTM.S39354 [Google Scholar] [CrossRef]
[30]. Javed N, Ghazanfar H, naseem s, Knowledge of dengue among students exposed to various awareness campaigns in model schools of islamabad: a cross-sectional studyCureus 2018 10(4):e2455-e2455.10.7759/cureus.2455 [Google Scholar] [CrossRef]
[31]. WHO. A Vision for Primary Health Care in The 21st Century [Internet]. Available from: https://www.who.int/docs/default-source/primary-health/vision.pdf [Google Scholar]
[32]. Carter JY, Lema OE, Wangai MW, Munafu CG, Rees PH, Nyamongo JA, Laboratory testing improves diagnosis and treatment outcomes in primary health care facilitiesAfrican Journal of Laboratory Medicine 2012 1(1):810.4102/ajlm.v1i1.829062728 [Google Scholar] [CrossRef] [PubMed]