Periodontitis is an inflammatory process of bacterial origin which affects the periodontium causing the loss of connective tissue attachment, loss of alveolar bone and migration of junctional epithelium. Due to plaque induced inflammation, the root surface gets modified which include changes like loss of collagen fiber insertion, contamination of root surface by endotoxins, increased mineral density and reduced chemotactic stimuli to the cells of the periodontal ligament which are responsible for regeneration [1]. Hence, periodontal therapy not only aims to arrest the disease but also to regenerate the lost periodontal structures which include the periodontal ligament, cementum and alveolar bone.
The first line of treatment for periodontal disease includes nonsurgical periodontal therapy, but the healing following the same is usually repair of the tissues rather than the regeneration. Hence, various regenerative techniques have been employed over the years which include the addition of bone graft, guided tissue regeneration, application of emdogain and combination techniques. Even though certain amount of regeneration is attained, the results are not very predictable [2].
Though mechanical instrumentation has been seen to remove the endotoxins on the root surface, but the root surface does not favour fibroblast attachment or connective tissue attachment because of presence of the smear layer, post-mechanical therapy. Hence the role of root biomodification was introduced wherein; an attempt was made to expose the collagen to enhance the chemo-attractant nature of the root surface by removing the smear layer [2].
The removal of smear layer has been attempted using root conditioning agent which began from the introduction of various acids. It was hypothesised that the success of root conditioning agents like citric acid was attributed to the fact that the acid caused exposure of collagen fibrils in the dentin matrix; this provided a suitable nidus for splicing with new fibrils during the healing process [3,4]. The exposure of the dentin matrix of root surface permits establishment of a proper fibrin clot which is a basis for the positive outcome of the early wound healing events [5], then enables the amalgamation between the root surface and the healing connective tissue favouring migration and attachment of gingival fibroblasts [6]. Thus, the use of chemical conditioning agents assists in root preparation combining the removal of the superficial mineral substance with the elimination of cytotoxic material and bacteria-derived products that affect root surfaces [7].
The popularity of acid demineralization began when Urist MR suggested that the dentin possessed inductive properties following citric acid demineralization [8]. Optimal cementogenesis and connective tissue attachment was seen with citric acid at the pH 1.0 for 2-3 minutes [8]. After which there was introduction of various root conditioning agents like Tetracycline HCl, EDTA, Maleic acid, etc., which were used in adjunct to mechanical therapy to enhance regeneration. These agents were thought to be advantageous as they remove the smear layer and make the root surface more suitable for periodontal regeneration. Etching agents, though have the property to alter the root surface but the primary disadvantage is the low pH which has a probability of damaging the surrounding periodontal tissues [6].
Hence there has been a search for a more biocompatible root biomodifier. CarisolvTM is a chemo-mechanical caries removal system to aid in the removal dentinal caries. It is a two-tube system with one of the component being sodium hypochlorite and the other being amino acids (lysine, leucine and glutamic acid). CarisolvTM when applied on oral mucosa for 3 minutes was seen to cause no or a weak inflammatory response that diminished after 72 hours [9].
EDTA is one of the traditionally used root conditioner which is known to have a neutral pH, hence does not harm the structure of the collagen fiber and periodontal structures. It exposes the collagen fibers for early cell migration and attachment of the periodontal ligament to the root surface. EDTA-S is a modification of EDTA in which Texapon, a derivative of sodium lauryl sulphate, is added to EDTA in 1:1 ratio. EDTA has a disadvantage of improper removal of smear layer which interfere in healing of the periodontal disease. This can be overcomed by the use of EDTA-S which has superior smear layer removal [10].
Materials and Methods
This in-vitro scanning electron microscopy study was conducted from June 2017 to January 2018 after obtaining ethical clearance from the ethical committee of the institution (IEC/REF/9306033). Sixty extracted teeth were collected from the Department of Oral and Maxillofacial Surgery, Sree Sai Dental College and Research Institute, Srikakulam, Andhra Pradesh.
Teeth included in the study were single rooted teeth, extracted teeth due to periodontal disease with normal root morphology, whereas, teeth with any pulpal involvement (caries), cervical abrasion, erosion and cervical restoration were excluded. These teeth were stored in saline till further treatment on the root surface.
The armamentarium included: Gracey’s Area Specific Curettes, Tweezers, Air rotor, Disk bur, Microtips, CarisolvTM, EDTA, EDTA-S, saline, 2% glutaraldehyde in 1.5M phosphate buffer, Graded alcohol solution [Table/Fig-1].
Armamentarium used in the study.

The teeth were initially delimited with a round bur to mark the area of interest. Then the selected teeth were randomly divided by simple random method of randomization into 4 groups with each group containing 15 samples (n=15) which were:
Group I-Scaling and Root Planing
In group I, scaling and root planing was performed with area specific curettes (Hu-Friedy, Chicago, IL, USA), using 30 strokes in the apico-coronal direction parallel to the long axis of the tooth.
Group II- CarisolvTM +Scaling and Root Planing
In group II, CarisolvTM (Medi Team, Dentalutveckling AB, Savedalen, Sweden) was applied prior to scaling and root planing for 30 seconds. This may facilitate the removal of calculus by chemically dissolving calculus and contaminated root surface. This is followed by 30 scaling and root planing strokes.
Group III- Scaling and Root Planing +15% EDTA
In group III, after 30 strokes of scaling and root planing with area specific curettes, EDTA is applied for about 2 minutes.
Group IV-Scaling and Root Planing +15% EDTA-S
EDTA-S is a modification of EDTA where it was added with Texapon (soft soap), a derivate of sodium lauryl sulphate, in 1:1 ratio. This was applied onto the tooth surface after 30 strokes scaling and root planing on the tooth surface for 2 minutes.
Preparation of Tooth Sections for Scanning Electron Microscopy
The root surface after the treatment with the root conditioners were rinsed with 20 mL of saline. The crowns were removed later at the cemento-enamel junction. The treated areas were sectioned horizontally and vertically with a diamond circular saw. Each tooth section was then rinsed in saline following which it was placed in 2.5% glutaraldehyde in 0.1 M phosphate buffer solution for 24 hours.
After removing from 2.5% glutaraldehyde solution in 0.1M phosphate buffer, the specimens were washed and dehydrated in graded alcohol solutions of 50%, 70%, 80%, 95% and 100% for 10 minutes each. After two additional washes with absolute alcohol, the specimens were left to dry in a desiccator jar overnight.
Sputter Coating
The teeth were removed from the desiccator jar and mounted on the SEM stubs [Table/Fig-2] and were gold coated with the help of a sputter coating machine [Table/Fig-3]. Sputtering adds a conducting surface (gold) to the non-conducting specimen for it to be recognised by a Scanning Electron Microscope.
Mounting of samples for sputter coating.


SEM Analysis
After sputtering, the sectioned samples were transferred into the scanning electron microscope [Table/Fig-4]. Every specimen was focused at the central portion with two magnifications, 1000X and 1500 X. The computer generated resultant image was then analysed [Table/Fig-5,6,7,8,9,10,11,12].
Mounting of samples in Scanning electron microscope.

Group I shows the incomplete removal of smear layer, blockage of the dentinal tubules with smear layer and an irregular surface; Also, the normal cementum or dentin cannot be appreciated due to the uniform distribution of smear layer on the root surface.
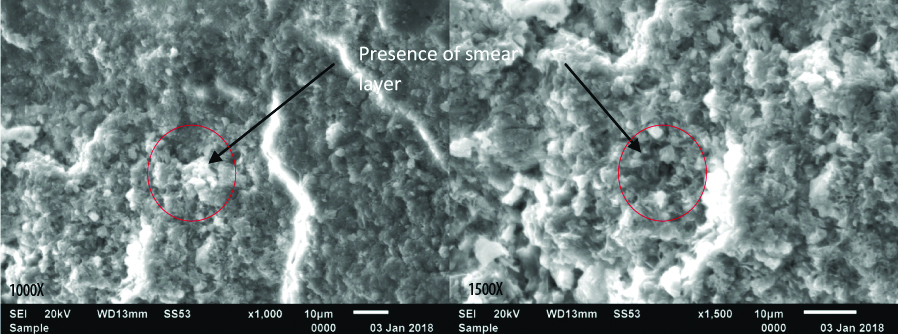
Group II shows almost complete removal of smear layer and no blockage of the dentinal tubules with smear layer; Though the smear layer has been removed and the underlying healthy cementum and dentin surfaces can be appreciated, the surface morphology is irregular due to the removal of cementum in certain areas.

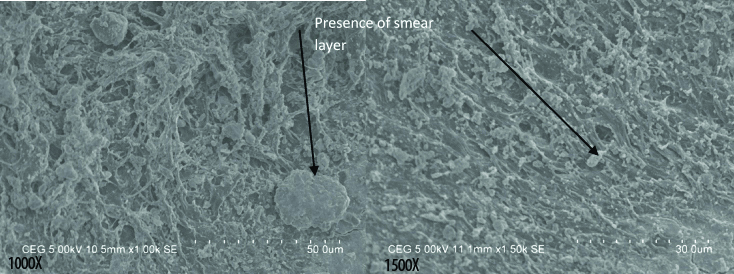
Group III shows uniformly distributed smear layer and blockage of the dentinal tubules with smear layer; Though the underlying tooth structures can be appreciated but they are uniformly covered with smear layer.
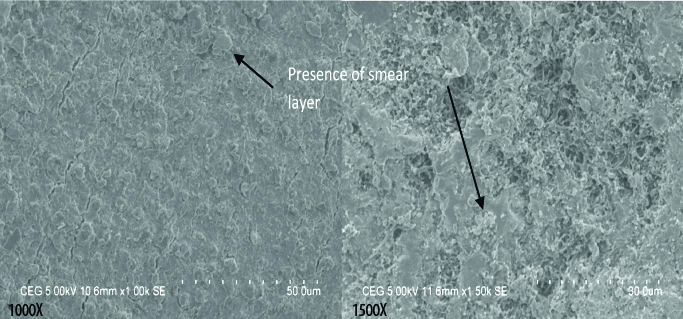
Group IV shows uniformly distributed smear layer and blockage of the dentinal tubules with smear layer; Though the underlying tooth structures can be appreciated but they are uniformly covered with smear layer.
The following parameters were examined by two examiners (SBP and RC) who were blinded for the root conditioning agent used with respect to the given sample:
Surface morphology whether regular or irregular [11];
Presence or absence of smear layer [11];
Patency of dentinal tubules [11];
Loss of tooth substance index (Lie T et al.,) [13]; and Sampaio’s Index for root surface modification (Sampaio JEC et al., 2005) [14].
The surface morphology, smear layer and dentinal tubules were evaluated by the two examiners where the interpretation was given as regular or irregular, present or not and patency of dentinal tubules respectively according to the SEM image so the evaluation was subjective. The reference of normal morphology of dental tubules was taken.
The scoring for Loss of tooth substance index [13] is as follows:
Score 0: Smooth and even root surface without marks from the instrumentation and with no loss of tooth substance.
Score 1: Slightly roughened or corrugated local areas confined to the cementum.
Score 2: Definitely corrugated local areas where the cementum may be completely removed, although most of the cementum is still present.
Score 3: Considerable loss of tooth substance with instrumentation marks into the dentin. The cementum is completely removed in large areas, or it has a considerable number of lesions from the instrumentation.
The scoring for Root Surface Modification Index [14] is as follows:
Score 1: Root surface without smear layer, with the dentinal tubules completely opened; no evidence of smear layer in the dentinal tubule gaps.
Score 2: Root surface without smear layer, with the dentinal tubules completely opened; evidence of smear layer in the dentinal tubule gaps.
Score 3: Root surface without smear layer, with the dentinal tubules partially opened.
Score 4: Root surface covered with smear layer, with uniform aspect; evidence of dentinal tubule gaps.
Score 5: Root surface covered with smear layer, with uniform aspect; no evidence of dentinal tubule gaps.
Score 6: Root surface covered with smear layer, with irregular aspect and presence of grooves and/or scattered debris.
Statistical Analysis
In the present study, the data was analysed statistically using SPSS version 23 for Windows. The Kappa statistics was used for calculating inter-observer variability. The non-parametric test, Kruskal Wallis test was used to assess the Loss of Tooth Substance Index and Root Surface Modification Index. Mann-Whitney U test was used to compare the various groups in Root Surface Modification Index.
Results
A total of 60 periodontally diseased teeth were extracted and included in the study. They were categorised into 4 treatment groups and were analysed under the scanning electron microscope for the following parameters.
Inter-examiner Variability
The Kappa agreement values obtained between the observers for various parameters were in the range of 0.5 to 1 which indicates that there is an acceptable agreement between the observers. Hence, the value of any one observer was taken into consideration [Table/Fig-13].
Analysis of inter observer agreement.
| Parameter | Group I | Group II | Group III | Group IV |
|---|
| 1000X | 1500X | 1000X | 1500X | 1000X | 1500X | 1000X | 1500X |
|---|
| Surface morphology | 0.571* | 1** | 1** | 1** | 1** | 1** | 1** | 1** |
| Smear layer | 1** | 1** | 1** | 0.865* | 1** | 1** | 1** | 1** |
| Patency of DT | 0.587* | 1** | 1** | 1** | 1** | 1** | 1** | 1** |
| LTSI | 0.628* | 0.874** | 1** | 0.826* | 1** | 1** | 1** | 1** |
| RSMI | 0.580* | 0.888** | 0.824** | 0.899** | 0.907** | 0.808** | 0.905** | 0.818** |
| 0-0.3 – low agreement0.3-0.79 – acceptable agreement0.8-0.99 – highly acceptable agreement1 – perfect agreement between the observers | DT – Dentinal tubulesLTSI – Loss of tooth substance indexRSMI – Root surface modification index*-acceptable or highly acceptable agreement**-Perfect agreement |
kappa Statistics
Surface Morphology
At 1000X, it was seen that the surface morphology was irregular in 66.7% and was regular in 33.3% of samples from Group I and Group II and 93.3% was irregular and 6.7% was regular in Group III and Group IV [Table/Fig-5,7,9,11].
At 1500X, it was seen that the surface morphology was irregular in 93.3% and 6.7% was regular in Group I, 80% was irregular and 20% was regular in Group II and 100% of the samples were irregular in Group III and Group IV [Table/Fig-6,8,10,12].
The study showed that Group III and Group IV were seen to have a more irregular surface as compared to Group I and Group II [Table/Fig-14].
Frequency distribution of Surface morphology in various groups.
| Magnification | Surface morphology | Group I | Group II | Group III | Group IV |
|---|
| N | % | N | % | N | % | N | % |
|---|
| 1000X | Irregular | 10 | 66.7 | 10 | 66.7 | 14 | 93.3 | 14 | 93.3 |
| Regular | 5 | 33.3 | 5 | 33.3 | 1 | 6.7 | 1 | 6.7 |
| 1500X | Irregular | 14 | 93.3 | 12 | 80.0 | 15 | 100.0 | 15 | 100.0 |
| Regular | 1 | 6.7 | 3 | 20.0 | 0 | 0.0 | 0 | 0.0 |
Smear Layer
At 1000X in Group I, smear layer was present in 86.7% and absent in 13.3%, Group II and Group III had smear layer in 66.7% samples and absent in 33.3% of samples and Group IV had 80% of samples with smear layer and 20% of samples without smear layer [Table/Fig-5,7,9,11].
At 1500X in Group I, smear layer was present in 93.3% and absent in 6.7% of samples, Group II had smear layer present in 60% of samples and absent in 40% of samples and Group III and Group IV had smear layer present in 86.7% samples and absent in 13.3% of samples [Table/Fig-6,8,10,12].
It was observed that all the groups had presence of smear layer with the maximum of smear layer present in Group I [Table/Fig-15].
Frequency distribution of Smear Layer in various groups.
| Magnification | Smear layer | Group I | Group II | Group III | Group IV |
|---|
| N | % | N | % | N | % | N | % |
|---|
| 1000X | Absent | 2 | 13.3 | 5 | 33.3 | 5 | 33.3 | 3 | 20.0 |
| Present | 13 | 86.7 | 10 | 66.7 | 10 | 66.7 | 12 | 80.0 |
| 1500X | Absent | 1 | 6.7 | 6 | 40.0 | 2 | 13.3 | 2 | 13.3 |
| Present | 14 | 93.3 | 9 | 60.0 | 13 | 86.7 | 13 | 86.7 |
Dentinal Tubules Patency
At 1000X in Group I, dentinal tubule patency was present in 46.7% and absent in 53.3%, Group II has dentinal tubules patency in 53.3% samples and absent in 46.7% of samples, Group III has patency in 40% samples and absent in 60% of samples and Group IV had 66.7% of samples had patency of dentinal tubules and 33.3% of samples had no patency [Table/Fig-5,7,9,11].
At 1500X in Group I, dentinal tubule patency was present in 40% and absent in 60%, Group II has dentinal tubules patency in 80% samples and absent in 20% of samples, Group III has patency in 46.7% samples and absent in 53.3% of samples and Group IV had 80% of samples had patency of dentinal tubules and 20% of samples had no patency [Table/Fig-6,8,10,12].
The study showed maximum amount of dentinal tubules patency in Group II and Group IV [Table/Fig-16].
Frequency distribution of Patency of Dentinal tubules in various groups.
| Magnification | Patency of dentinal tubules | Group I | Group II | Group III | Group IV |
|---|
| N | % | N | % | N | % | N | % |
|---|
| 1000X | Absent | 8 | 53.3 | 7 | 46.7 | 9 | 60.0 | 5 | 33.3 |
| Present | 7 | 46.7 | 8 | 53.3 | 6 | 40.0 | 10 | 66.7 |
| 1500X | Absent | 9 | 60.0 | 3 | 20.0 | 8 | 53.3 | 3 | 20.0 |
| Present | 6 | 40.0 | 12 | 80.0 | 7 | 46.7 | 12 | 80.0 |
Loss of Tooth Substance Index (LTSI)
The scores indicated that the majority of the samples in all the four groups had a corrugated surface with local areas of partial removal of cementum. At 1000X and 1500X, between the scores of LTSI there was no statistical difference (p>0.05) amongst the various groups. Hence the loss of tooth substance in all the groups is the same [Table/Fig-17].
Frequency distribution according to Loss of Tooth Surface Index Score in various groups.
| Magnification | Score | Group I | Group II | Group III | Group IV | H value | p-value |
|---|
| N | % | N | % | N | % | N | % |
|---|
| 1000X | Score 1 | 4 | 26.7 | 3 | 20.0 | 2 | 13.3 | 2 | 13.3 | 0.317 | 0.813NS |
| Score 2 | 9 | 60.0 | 10 | 66.7 | 11 | 73.3 | 10 | 66.7 |
| Score 3 | 2 | 13.3 | 2 | 13.3 | 2 | 13.3 | 3 | 20.0 |
| Median | 2 | 2 | 2 | 2 |
| 1500X | Score 1 | 4 | 26.7 | 1 | 6.7 | 2 | 13.3 | 1 | 6.7 | 0.812 | 0.493NS |
| Score 2 | 9 | 60.0 | 12 | 80.0 | 12 | 80.0 | 11 | 73.3 |
| Score 3 | 2 | 13.3 | 2 | 13.3 | 1 | 6.7 | 3 | 20.0 |
| Median | 2 | 2 | 2 | 2 |
Kruskal Wallis Test
Root Surface Modification Index (RSMI)
There was high statistically significant difference (p<0.001) present in the RSMI scores in various groups at different magnifications [Table/Fig-6]. The individual group comparison was done using Mann-Whitney U Test. The RSMI scores showed statistical significance between Group I and Group II, Group II and Group III and Group II and Group IV (p<0.001) at 1000X and 1500X magnification. There was no statistical significance in the RSMI scores between Group I and Group III, Group I and Group IV and Group III and Group IV [Table/Fig-18].
Frequency distribution according to Root Surface Modification Index Score in various groups.
| Magnification | Score | Group I | Group II | Group III | Group IV | H value | p-value |
|---|
| N | % | N | % | N | % | N | % |
|---|
| 1000X | Score 1 | 1 | 6.7 | 5 | 33.3 | 0 | 0.0 | 0 | 0.0 | 18.320 | <0.001** |
| Score 2 | 1 | 6.7 | 4 | 26.7 | 0 | 0.0 | 0 | 0.0 |
| Score 3 | 0 | 0 | 3 | 20.0 | 4 | 26.7 | 3 | 20.0 |
| Score 4 | 2 | 13.3 | 1 | 6.7 | 3 | 20.0 | 6 | 40.0 |
| Score 5 | 9 | 60.0 | 2 | 13.3 | 6 | 40.0 | 4 | 26.7 |
| Score 6 | 2 | 13.3 | 0 | 0.0 | 2 | 13.3 | 2 | 13.3 |
| Median | 5 | 2 | 5 | 4 |
| 1500X | Score 1 | 0 | 0.0 | 7 | 46.7 | 0 | 0.0 | 0 | 0.0 | 25.003 | <0.001** |
| Score 2 | 0 | 0.0 | 5 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| Score 3 | 1 | 6.7 | 1 | 6.7 | 2 | 13.3 | 2 | 13.3 |
| Score 4 | 4 | 26.7 | 1 | 6.7 | 6 | 40.0 | 4 | 26.7 |
| Score 5 | 9 | 60.0 | 0 | 0.0 | 5 | 33.3 | 4 | 26.7 |
| Score 6 | 1 | 6.7 | 1 | 6.7 | 2 | 13.3 | 5 | 33.3 |
| Median | 5 | 2 | 4 | 5 |
**- Highly significant (p<0.001); Kruskal Wallis Test
The RSMI scores in Group II have a median of 2 at both 1000X and 1500X magnification indicating the better root biomodification properties of CarisolvTM [Table/Fig-19].
Individual Pair wise comparisons of scores of Root Surface Modification Index.
| Comparison Between | 1000X | | | 1500X | | |
|---|
| Difference | z-value | p-value | Difference | z-value | p-value |
|---|
| Group I | Group II | 3 | -3.341 | 0.001* | 3 | -4.018 | <0.001** |
| Group I | Group III | 0 | -0.735 | 0.512NS | 1 | -0.76 | 0.486NS |
| Group I | Group IV | 1 | -1.16 | 0.285NS | 0 | -0.483 | 0.653NS |
| Group II | Group III | 3 | -3.429 | <0.001** | 2 | -3.907 | <0.001** |
| Group II | Group IV | 2 | -3.378 | <0.001** | 3 | -4.006 | <0.001** |
| Group III | Group IV | 1 | -0.238 | 0.838NS | 1 | -0.929 | 0.389NS |
(Mann-Whitney U test)
Discussion
Regenerative periodontal therapy utilises various biomaterials in the form of bone grafts, guided tissue regeneration, emdogain, etc. However, all these cannot be integrated with the root due to the presence of smear layer which acts as a physical barrier between the root and the periodontium and inhibits the formation of new attachment [15,16]. Smear layer is formed by residual calculus, microorganisms and their endotoxins, cementum and dentin fragments [17]. In an attempt to eliminate this smear layer before any regenerative procedure, root biomodification agents are being used for the past several decades. Some of the procedures to detoxify the root surface recommended are acid etching. By definition, etching involves selective removal of part or component from a solid surface through the action of etching agents such as solutions of acids and other substances. Etching does not, however, imply erosion of the surface or removal of a complete surface layer. Apart from etchants, certain other detoxifying agents like Citric acid, Tetracycline HCl, Maleic acid, EDTA, etc., were used which were primarily acids and which can influence the vitality of surrounding periodontal tissues [18].
In addition, the above mentioned biomodification agents failed to show an added benefits in periodontal regeneration [19]. The drawbacks of the above mentioned root bio-modifiers led to the advent of new root bio-modification agents that can be more biocompatible and show better results in periodontal regeneration.
The usage of chemical agents in association with mechanical treatment epitomises a possibility of a less traumatic procedure, averting the excessive loss of root substance. CarisolvTM is one such agent that has been introduced in the recent past. In the field of Periodontics, the possibility of chemically dissolving calculus and contaminated root cementum in order to enable their mechanical removal is one of the most promising applications of CarisolvTM gel [11]. Additionally, it can remove the smear layer during the mechanical removal as it will act as a lubricating gel. CarisolvTM gel was also able to remove the contaminated cementum layer and expose the healthy structure [12]. Also, CarisolvTM has been seen to have bactericidal action against Streptococcus mutans and Lactobacillus species [20]. Therefore the current study has used mildly alkaline root conditioner in the form of CarisolvTM which has been found to have minimal effect on the soft tissue [9].
EDTA, a chelating agent, which works at a neutral pH has been preferred over low pH acidic agents as it preserves the integrity of the exposed collagen fibers, early cell colonisation, and periodontal wound healing. In addition, etching at neutral pH has been reported to preserve adjacent tissue-vitality, while etching at low pH necrotizes the flap and adjacent periodontium after 20 seconds of exposure [6].
EDTA-S is known to deliver better smear layer removal than EDTA alone. Soft soap is extensively used in the medical field to eliminate incrustation in scaly skin diseases; it is also used in solution form with warm water as an enema and this implies its reaction with the mucous membranes and degree of safety. The addition of soft soap, a tense active detergent, decreases the surface strain.
The current in-vitro scanning electron microscopic study, compared the effect of CarisolvTM, EDTA and EDTA-S as root biomodifiers on periodontally diseased root surface. This study aimed to identify a non-acidic form of root conditioning agents which will help in the removal of smear layer and also do not cause any effect on the adjacent tissues during application of the agent. All the three selected root conditioning agents were mildly alkaline and proven to have minimal soft tissue effects [4,11]. To the best of our knowledge, this is the first study which compares these three root conditioning agents for the first time.
The parameters and the indices were evaluated by 2 blinded examiners. Inter-observer variability was analysed using Kappa statistics and an acceptable agreement was seen. It was seen that most of the surfaces had an irregular surface morphology. The presence of irregular root surface may be seen due to the scaling and root planing done on the root surface which may appear irregular at a microscopic level and/or the incomplete removal of smear layer. In the present study, it was seen that a relatively higher number of samples in EDTA and EDTA-S groups showed an irregular surface at both 1000X and 1500X magnification.
The presence of smear layer was primarily seen in scaling and root planing group in both 1000X and 1500X magnification which was in accordance with a study by Polson AM et al., [15]. This was followed by EDTA and EDTA-S which is also supported by a previous study performed by Sampaio JEC et al., where both the agents were unable to remove the smear layer effectively when they were applied passively on the tooth surface. The smear layer removal was satisfactory when actively applied [17]. A study conducted by Bergentoitz A et al., had observed that there is disruption of collagenous fibers and severe etching of cementum on active burnishing of root conditioning agents [21]. Hence in the current study passive application has been done.
In the present study, specimens treated with CarisolvTM showed a mosaic like appearance depicting the cementum, which indicates that by the use of chemo-mechanical therapy there was removal of the diseased cementum and exposure of the healthy cementum. This was in agreement with a previous study performed by Grisi DC et al., [11]. This flaky surface is seen due to the lack of smear layer which is in accordance with a study performed by Banerjee A et al., [12].
Dentinal tubule patency was seen in CarisolvTM group in both the magnification. This was in accordance with a study conducted by Banerjee A et al., where the dentinal tubule patency was seen even at 200X magnification [12]. This was not seen in accordance with studies performed by Grisi DC et al., and Gohil M et al., where CarisolvTM was stated as an agent that will act as an adjunct to SRP which will cause in chemo-mechanical removal of the calculus rather than act as an etchant to modify the root surface [11,22]. In both the studies it was seen that there was dentinal tubules patency achieved on active application of CarisolvTM, but it was not statistically significant. The patency of dentinal tubules was attributed to the mechanical burnishing of the agent using microbrush which increased the effect on the root surface.
Patency of dentinal tubules was seen to be minimal in control group and samples treated with EDTA in both 1000X and 1500X magnification. The results corresponding to scaling and root planing was in agreement with the previous studies performed by Blomlöf J et al., where citric acid, phosphoric acid and EDTA was compared with scaling and root planing alone in modifying the texture [18]. In case of EDTA similar results were seen in a study conducted by Amaral NG et al., where they evaluated the demineralizing effect of Citric acid, tetracycline, phosphoric acid and EDTA [23].
In the current study, the loss of tooth substance index was evaluated between the four groups. Loss of tooth substance indicates the mode of action and force applied by the practitioner, the angulation and the sharpness of the curettes [13]. This index was taken to standardise all the above parameters and to eliminate any bias in difference of instrumentation. As there was no statistical significance seen between the four groups, the bias was eliminated.
The current study is the first study which has used the Root Surface Biomodification Index on CarisolvTM. Also it is the first study comparing CarisolvTM with EDTA and EDTA-S. In the present study for better evaluation of the root surface, post-modification was done to use the root surface modification index proposed by Sampaio JEC et al [17]. This index describes all the aspects in relation to the smear layer and its presence in and around the dentinal tubules.
In this study, CarisolvTM was seen to have a statistically significant root biomodification property on comparing the Root Surface Modification index at both 1000X and 1500X magnification. It was seen to have a mean score of 2 which interprets that the root surface is without smear layer, with dentinal tubules completely opened; evidence of smear layer in the dentinal tubule gaps. This corresponds with the findings of the studies conducted by Banerjee A et al., and Gohil M et al., [12,22]. These results are also supported by studies conducted by Grisi DC et al., where there was removal of smear layer and opening of dentinal tubules on multiple application but not superior to 24% EDTA [11].
When EDTA was evaluated there was no significant difference in the root biomodification properties as compared to SRP. This corresponds to the previous in-vitro and in-vivo studies and systematic reviews which has stated that there was no significant difference in the healing of the tooth treated with or without EDTA [19,24,25]. In the in-vivo studies, although the collagen exposure by root biomodification promoted the adhesion of fibrin clots, EDTA is seen to have a chelating effect on the blood which has an inhibitory effect on the coagulation cascade. This may be a probable reason for the impairment of the effects of EDTA when used as a root biomodification agent [19].
EDTA-S also did not show any significant root biomodification properties as compared to the other 3 groups, which is in accordance with a study conducted by Sampaio JEC et al., [17]. Though not significant a mild superiority as compared to the SRP and EDTA was seen at 1000X magnification according to the RSMI scores. This corresponds to results obtained in previous studies [10,26]. EDTA-S may have failed to show statistical difference because of passive application of EDTA-S as opposed to the active application in the previous study [26].
It was seen that in 1000X magnification when SRP, EDTA and EDTA-S were compared, EDTA-S had a better RSMI score and at 1500X, it was seen that EDTA was superior but these values were not statistically significant. This ambiguity in different magnifications may be due to the area of focus of the samples which may or may not visualise the areas with the smear layer.
Recommendations for Further Work
Root biomodification should be added as a mandatory step in periodontal therapy as it was seen to improve the properties of the root surface to accept the new repopulating cells. CarisolvTM, a chemo-mechanical agent when used along with SRP, has proven to reduce the amount of smear layer in the previous studies and the present study. Hence, it is recommended to evaluate its efficacy under clinical conditions as well.
Limitation
One of the limitations of our study was the small sample size. The other aspect is that, studies which have shown positive results in-vitro have occasionally failed to show similar results in clinical trials. Hence in-vivo studies must be conducted to confirm the same. Lastly, the cost of CarisolvTM is high and has a limited shelf life period of 1 year.
Conclusion
CarisolvTM was seen to have better root biomodification properties as compared to the other groups. Hence there is requirement to evaluate the same under clinical situations. All the agents used in the study have shown to be biologically acceptable, hence clinical trials are suggested to confirm the results clinically. Also, certain studies indicate the active application of root conditioning agents with the help of microtip which also should be evaluated.